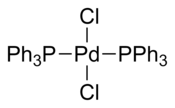
Bis(triphenylphosphine)palladium chloride
It is a yellow solid that is soluble in some organic solvents.
This compound may be prepared by treating palladium(II) chloride with triphenylphosphine:[2][3] Upon reduction with hydrazine in the presence of excess triphenylphosphine, the complex is a precursor to tetrakis(triphenylphosphine)palladium, Pd(PPh3)4:[4] Several crystal structures containing PdCl2(PPh3)2 have been reported.
In all of the structures, PdCl2(PPh3)2 adopts a square planar coordination geometry and the trans isomeric form.
[5][6][7][8] The complex is used as a pre-catalyst for a variety of coupling reactions.
Using bis(triphenylphosphine)palladium chloride as the catalyst, triflates and boronic acids have been coupled on an 80 kilogram scale in good yield.