Suzuki reaction
[1][2][3] It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of noble metal catalysis in organic synthesis.
The general scheme for the Suzuki reaction is shown below, where a carbon–carbon single bond is formed by coupling a halide (R1-X) with an organoboron species (R2-BY2) using a palladium catalyst and a base.
Reaction (metathesis) with base gives intermediate C, which via transmetalation[8] with the boron-ate complex D (produced by reaction of the boronic acid reagent 2 with base) forms the transient organopalladium species E. Reductive elimination step leads to the formation of the desired product 3 and restores the original palladium catalyst A which completes the catalytic cycle.
The base was first believed to form a trialkyl borate (R3B-OR), in the case of a reaction of a trialkylborane (BR3) and alkoxide (−OR); this species could be considered as being more nucleophilic and then more reactive towards the palladium complex present in the transmetalation step.
[9][10][11] Duc and coworkers investigated the role of the base in the reaction mechanism for the Suzuki coupling and they found that the base has three roles: Formation of the palladium complex [ArPd(OR)L2], formation of the trialkyl borate and the acceleration of the reductive elimination step by reaction of the alkoxide with the palladium complex.
In the case of the Suzuki coupling the ligands are transferred from the organoboron species D to the palladium(II) complex C where the base that was added in the prior step is exchanged with the R2 substituent on the organoboron species to give the new palladium(II) complex E. The exact mechanism of transmetalation for the Suzuki coupling remains to be discovered.
Phosphine ligand increases the electron density at the metal center of the complex and therefore helps in the oxidative addition step.
[24] Another example is the coupling of 3-pyridylborane and 1-bromo-3-(methylsulfonyl)benzene that formed an intermediate that was used in the synthesis of a potential central nervous system agent.
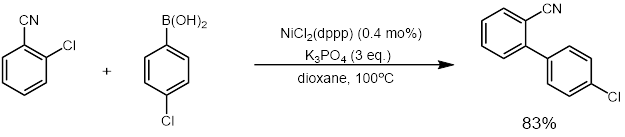
[26][27] The Suzuki coupling has been used on a citronellal derivative for the synthesis of caparratriene, a natural product that is highly active against leukemia:[28] Various catalytic uses of metals other than palladium (especially nickel) have been developed.
[29] The use of nickel catalysts has allowed for electrophiles that proved challenging for the original Suzuki coupling using palladium, including substrates such as phenols, aryl ethers, esters, phosphates, and fluorides.
[29] Investigation into the nickel catalyzed cross-coupling continued and increased the scope of the reaction after these first examples were shown and the research interest grew.
Miyaura and Inada reported in 2000 that a cheaper nickel catalyst could be utilized for the cross-coupling, using triphenylphosphine (PPh3) instead of the more expensive ligands previously used.
[29] Advancements by Han and co-workers have tried to address that problem by developing a method using low amounts of nickel catalyst (<1 mol%) and no additional equivalents of ligand.
Apart from Pd and Ni catalyst system, cheap and non-toxic metal sources like iron and copper[34] have been used in Suzuki coupling reaction.
[38] The synthesis of a tubulin-binding compound (antiproliferative agent) was carried out using a trimethoxybenzamide and an indolyl pinacolatoboron coupling partner on a gram scale.
[39] Aryltrifluoroborate salts can be formed from boronic acids by the treatment with potassium hydrogen fluoride which can then be used in the Suzuki coupling reaction.
[42] This increased the scope of coupling reactions, as a variety of water-soluble bases, catalyst systems, and reagents could be used without concern over their solubility in organic solvent.