Bisphosphonate
Bisphosphonates are a class of drugs that prevent the loss of bone density, used to treat osteoporosis and similar diseases.
The use of strontium ranelate is restricted because of increased risk of venous thromboembolism, pulmonary embolism and serious cardiovascular disorders, including myocardial infarction.
In 2009 bisphosphonates were "among the only class of medications that has survived placebo-controlled studies showing statistically significant improvement (in CRPS) with therapy.
[22][23] Bisphosphonates have been used to reduce fracture rates in children with the disease osteogenesis imperfecta[24] and to treat otosclerosis[25] by minimizing bone loss.
Intravenous bisphosphonates can give fever and flu-like symptoms after the first infusion, which is thought to occur because of their potential to activate human γδ T cells.
[26] A number of cases of severe bone, joint, or musculoskeletal pain have been reported, prompting labeling changes.
[27] Some studies have identified bisphosphonate use as a risk factor for atrial fibrillation (AF), though meta-analysis of them finds conflicting reports.
As of 2008[update], the US Food and Drug Administration did not recommend any alteration in prescribing of bisphosphonates based on AF concerns.
It is hypothesized that micro-cracks in the bone are unable to heal and eventually unite and propagate, resulting in atypical fractures.
[31][32][non-primary source needed] In cases where there is concern of such fractures occurring, teriparatide is potentially a good alternative because it does not cause as much damage as a bisphosphonate does by suppressing bone turnover.
The long side-chain (R2 in the diagram) determines the chemical properties, the mode of action and the strength of bisphosphonate drugs.
Of the bisphosphonate that is resorbed (from oral preparation) or infused (for intravenous drugs), about 50% is excreted unchanged by the kidney.
The specificity of bisphosphonate-based drugs comes from the two phosphonate groups (and possibly a hydroxyl at R1) that work together to coordinate calcium ions.
Bisphosphonate molecules then attach to and enter osteoclasts where they disrupt intracellular enzymatic functions needed for bone resorption.
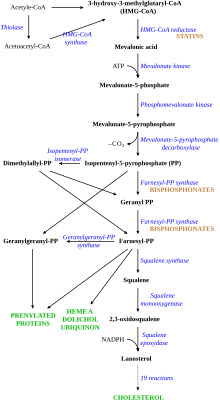
[41] Disruption of the HMG CoA-reductase pathway at the level of FPPS prevents the formation of two metabolites (farnesol and geranylgeraniol) that are essential for connecting some small proteins to the cell membrane.
In particular, the cytoskeleton is vital for maintaining the "ruffled border" that is required for contact between a resorbing osteoclast and a bone surface.
Nevertheless, some studies have reported a decreased rate of fracture (an indicator of osteoporosis) and/or an increased bone mineral density in statin users.