Bombykol
[3] Minute quantities of this pheromone can be used per acre of land to confuse male insects about the location of their female partners.
It can thus serve as a lure in traps to remove insects effectively without spraying crops with large amounts of pesticides.
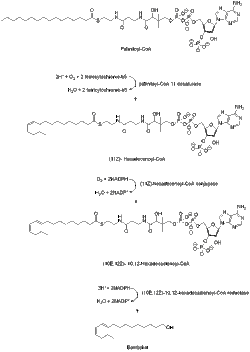
[7] Compared to other Type I pheromones, bombykol biosynthesis does not need chain-shortening or any other kind of modification of the terminal hydroxyl group.
[8] The bombykol acyl precursor (10E,12Z)-10,12-hexadecadienoate is primarily found as a triacylglycerol ester in the cytoplasmic lipid droplets of pheromone gland cells of the moth.
And when the adult females emerge from their pupae, the neurohormone PBAN (pheromone biosynthesis-activating neuropeptide) start signaling events that help control the lipolysis of the stored triacylglycerols, releasing (10E,12Z)-10,12-hexadecadienoate for its final reductive modification.