Bottromycin
[1] It has been shown to inhibit methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) among other Gram-positive bacteria and mycoplasma.
[3] Although bottromycin exhibits antibacterial activity in vitro, it has not yet been developed as a clinical antibiotic, potentially due to its poor stability in blood plasma.
Bottromycin falls within the ribosomally synthesized and post-translationally modified peptide class of natural product.
[8] Bottromycin has a unique structure, consisting of the macrocyclic amidine linkage and four β-methylated amino acids.
[2] However, the unique structure and mode of action have recently made bottromycin a more target for drug development, especially given the rise of antibiotic resistance.
[citation needed] Bottromycin is produced naturally as a series of products differing in methylation patterns.
Its peptide-like structure, including the presence of glycine and valine, was first suggested by a combination of acidic hydrolysis, acetylation, ninhydrin staining, and paper chromatography, among other experiments.
[9] The presence of a thiazole ring, along with an adjacent β-methylated phenylalanine, was established by ninhydrin staining, potassium permanganate oxidation, and comparison to synthetic standards.
[11] The same study also reported that the Kunz hydrolysis product lacking a methyl ester was biologically inactive.
The structure was confirmed in the 1980s and 1990s to be a cyclic iminopeptide based on NMR studies, with a linear side chain connected to the macrocycle via an amidine linkage.
Stereochemistry at carbon 43 was confirmed by comparing 1H NMR of authentic hydrolysis product to a chemically synthesized sample of the same fragment.
The methylated proline and β-OMe alanine residues were found to be on the same face of bottroymycin A2 and it was suggested that this characteristic contributed to binding of bottromycin to the ribosomal A site.
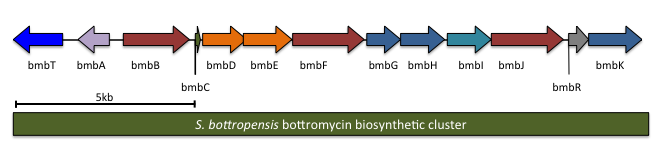
[6][7][16][17] It was independently confirmed in 2012 by multiple groups that bottromycin is produced as a ribosomal peptide natural product that it subsequently post-translationally modified.
Ribosomal peptide synthesis, which is the same machinery that produces all proteins found in the cell, is limited to the 20 proteinogenic amino acids.
However, bottromycin was found to be a highly modified ribosomal peptide by a combination of genome mining and gene deletion studies.
It is predicted to contain 13 genes, including the precursor peptide (notation will follow Crone and colleagues;[7] other studies had similar results).
btmM, with homology to Zn+2 aminopeptidases, is predicted to cleave the N-terminal methionine residue, which is not present in the bottromycin final product.
The radical SAM methyltransferases are believed to β-methylate amino acid residues within the precursor peptide.
Although the evidence points to radical β-methylation during bottromycin biosynthesis, it remains to be seen whether bioinformatic hypothesis and feeding studies will be supported by in vitro activity assays.
As such, there are still three predicted radical SAM dependent enzymes in the bottromycin D biosynthetic cluster: bstC, bstF, and bstJ.
To obtain the primary thia-β-Ala-OMe intermediate, a sequence of condensation, Mannich reaction, and palladium-catalyzed decarboxylation steps were performed.
Further, the synthetic sample of bottromycin was also found to have antibacterial activity against both MRSA and VRE, although quantitative data was not reported.
Unlike the previous synthesis, Ackerman and colleagues synthesized a linear peptide and achieved intramolecular amidine formation using an S-methylated endothiopeptide.
Bottromycin A2 had low micromolar activity against all the strains tested, ranging from an MIC of 0.5 μg/mL in E. faecalis NCTC12203 to 2 μg/mL in MRSA HH-1.
The amide and urea derivative families were found to have weaker antibacterial activity than bottromycin A2 against S. aureus, MRSA, and VRE.
Bottromycin A2 completely degraded in mouse plasma after 10 minutes and exhibited 0% residual activity after exposure to rat serum.
In contrast, many derivatives retained a significant percentage of residual anti-MRSA activity following exposure to serum.
A combination of biologic and synthetic techniques may yield both an efficacious and stable bottromycin analog for development as a potential drug candidate.