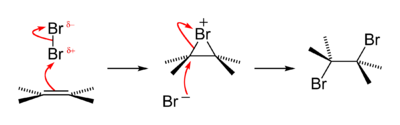Bromine
It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour.
Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived from Ancient Greek βρῶμος (bromos) 'stench', referring to its sharp and pungent smell.
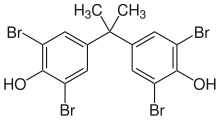
This effect makes organobromine compounds useful as fire retardants, and more than half the bromine produced worldwide each year is put to this purpose.
Bromine compounds are still used in well drilling fluids, in photographic film, and as an intermediate in the manufacture of organic chemicals.
[11] Hundreds of known organobromine compounds are generated by terrestrial and marine plants and animals, and some serve important biological roles.
[14][20][18][21] Other sources claim that the French chemist and physicist Joseph-Louis Gay-Lussac suggested the name brôme for the characteristic smell of the vapors.
[22][23] Bromine was not produced in large quantities until 1858, when the discovery of salt deposits in Stassfurt enabled its production as a by-product of potash.
In 1840, bromine was discovered to have some advantages over the previously used iodine vapor to create the light sensitive silver halide layer in daguerreotypy.
[25] By 1864, a 25% solution of liquid bromine in .75 molar aqueous potassium bromide[26] was widely used[27] to treat gangrene during the American Civil War, before the publications of Joseph Lister and Pasteur.
Like all halogens, it is thus one electron short of a full octet, and is hence a strong oxidising agent, reacting with many elements in order to complete its outer shell.
[31] These similarities led to chlorine, bromine, and iodine together being classified as one of the original triads of Johann Wolfgang Döbereiner, whose work foreshadowed the periodic law for chemical elements.
[31] The halogens darken in colour as the group is descended: fluorine is a very pale yellow gas, chlorine is greenish-yellow, and bromine is a reddish-brown volatile liquid that freezes at −7.2 °C and boils at 58.8 °C.
Br isotopes from 87Br and heavier undergo beta decay with neutron emission and are of practical importance because they are fission products.
Anhydrous hydrogen bromide is a poor solvent, only able to dissolve small molecular compounds such as nitrosyl chloride and phenol, or salts with very low lattice energies such as tetraalkylammonium halides.
The exceptions are decidedly in the minority and stem in each case from one of three causes: extreme inertness and reluctance to participate in chemical reactions (the noble gases, with the exception of xenon in the very unstable XeBr2); extreme nuclear instability hampering chemical investigation before decay and transmutation (many of the heaviest elements beyond bismuth); and having an electronegativity higher than bromine's (oxygen, nitrogen, fluorine, and chlorine), so that the resultant binary compounds are formally not bromides but rather oxides, nitrides, fluorides, or chlorides of bromine.
[41] The halogens form many binary, diamagnetic interhalogen compounds with stoichiometries XY, XY3, XY5, and XY7 (where X is heavier than Y), and bromine is no exception.
It reacts violently with water and explodes on contact with flammable materials, but is a less powerful fluorinating reagent than chlorine trifluoride.
[49] More important are the bromates, which are prepared on a small scale by oxidation of bromide by aqueous hypochlorite, and are strong oxidising agents.
The Br–O bond in BrO−4 is fairly weak, which corresponds to the general reluctance of the 4p elements arsenic, selenium, and bromine to attain their group oxidation state, as they come after the scandide contraction characterised by the poor shielding afforded by the radial-nodeless 3d orbitals.
[53] Bromine is significantly less abundant in the crust than fluorine or chlorine, comprising only 2.5 parts per million of the Earth's crustal rocks, and then only as bromide salts.
Salt lakes and brine wells may have higher bromine concentrations: for example, the Dead Sea contains 0.4% bromide ions.
Epoxies used in printed circuit boards are normally made from such flame retardant resins, indicated by the FR in the abbreviation of the products (FR-4 and FR-2).
[65] A number of gaseous or highly volatile brominated halomethane compounds are non-toxic and make superior fire suppressant agents by this same mechanism, and are particularly effective in enclosed spaces such as submarines, airplanes, and spacecraft.
[71] Commercially available organobromine pharmaceuticals include the vasodilator nicergoline, the sedative brotizolam, the anticancer agent pipobroman, and the antiseptic merbromin.
[51] Other uses of organobromine compounds include high-density drilling fluids, dyes (such as Tyrian purple and the indicator bromothymol blue), and pharmaceuticals.
Bromine is used in cooling towers (in place of chlorine) for controlling bacteria, algae, fungi, and zebra mussels.
[74] A 2014 study suggests that bromine (in the form of bromide ion) is a necessary cofactor in the biosynthesis of collagen IV, making the element essential to basement membrane architecture and tissue development in animals.
While significant and sometimes serious disturbances occur to neurologic, psychiatric, dermatological, and gastrointestinal functions, death from bromism is rare.
[83] Caution is required when transporting bromine; it is commonly carried in steel tanks lined with lead, supported by strong metal frames.
[61] The Occupational Safety and Health Administration (OSHA) of the United States has set a permissible exposure limit (PEL) for bromine at a time-weighted average (TWA) of 0.1 ppm.