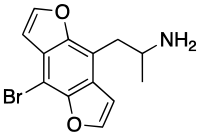
Bromo-DragonFLY
[3] As with the earlier and less potent dihydrofuran series of compounds nicknamed FLY, Bromo-DragonFLY was named after its superficial structural resemblance to a dragonfly.
The first synthesis of racemic Bromo-DragonFLY was reported by David E. Nichols in 1998 and was an expansion upon earlier research into the tetrahydrobenzodifuran analogue of DOB.
[3] The 1998 synthesis of racemic Bromo-DragonFLY starts from hydroquinone, which is dialkylated with 1-bromo-2-chloroethane, brominated, and treated with n-butyllithium to yield the tetrahydrobenzodifuran ring system.
The nitropropene derivative was then reduced with lithium aluminium hydride to yield the amine intermediate, which was protected with trifluoroacetic anhydride.
Following para-bromination with elemental bromine and oxidation of the tetrahydrobenzodifuran ring system with DDQ, the trifluoroacetyl protecting group of the amine was removed to give Bromo-DragonFLY as a racemic mixture of the R and S enantiomers.
To synthesize the more active R enantiomer, a derivative of D-alanine was reacted with 2,3,6,7-tetrahydrobenzodifuran in a Friedel–Crafts acylation, yielding an intermediate containing a β-keto moiety which was removed by treatment with triethylsilane in trifluoroacetic acid.
Data on toxicological significance and dosage of Bromo-DragonFLY remains elusive due to lack of human consumption, however commonly reported recreational dose of this substance is in the range of 500–1000μg.
Laboratory testing has confirmed that in October 2009, a batch of Bromo-Dragonfly was distributed, mislabeled as the related compound 2C-B-FLY, which is around 20x less potent than BDF by weight.
In September 2007, a 35-year-old Swedish male required amputation of the front part of his feet and several fingers on one hand after taking a massive (but unknown) overdose; reportedly, the compound acted as a long-acting efficacious vasoconstrictor, leading to necrosis and gangrene which became apparent several weeks after the overdose occurred.
[21] As of Oct 12, 2016, Bromo-DragonFLY is listed in Schedule III of the Canadian Controlled Drugs and Substances Act: "2C-phenethylamines and their salts, derivatives, isomers and salts of derivatives and isomers", a broad definition which corresponds to anything with a 2,5-dimethoxyphenethylamine core, including (but not limited to) the 2C family (including e.g. βk-2C-B), the DOx chemical class, the TMA family, Aleph aka DOT, NBOMe, the 25x-NBx series, and of course, Bromo-DragonFLY itself (see this article).
[22] However, as of 2014, it remains unclear to what extent it is covered by the UK phenylethylamine catch-all clause, with commentators noting both the structural similarities[23] and differences[24][unreliable source?]