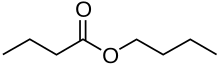
Butyl butyrate
Butyl butyrate, or butyl butanoate, is an organic compound that is an ester formed by the condensation of butyric acid and n-butanol.
It is a clear, colorless liquid that is insoluble in water, but miscible with ethanol and diethyl ether.
Like other volatile esters, butyl butyrate has a pleasant aroma.
It occurs naturally in many kinds of fruit including apple, banana, berries, pear, plum, and strawberry.
[2] It mildly irritates the eyes and skin.