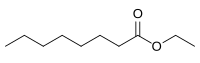
Ethyl octanoate
[5] Ethyl octanoate can be synthesized from caprylic acid and ethanol via a classic Fischer–Speier esterification.
Equilibrium can be shifted towards the right side of the equation through removal of water.
Ethyl octanoate has a strong odor of fruit and flowers and a taste of apricot, and as such it can be used as a flavoring or to create scents.
It is found in some wines, where overall ester concentration and composition is considered important to the flavor and aroma profile.
Though it has a low explosive limit of only 0.67 vol%, it is also weakly volatile, with a vapor pressure of only 0.2 mbar at room temperature.