CAR T cell
[10] Originally termed "T-bodies", these early approaches combined an antibody's ability to specifically bind to diverse targets with the constant domains of the TCR-α or TCR-β proteins.
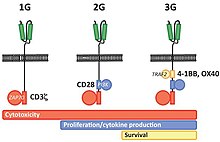
Termed second generation CARs, these constructs showed greater persistence and improved tumor clearance in pre-clinical models.
[16] Clinical trials in the early 2010s using second generation CARs targeting CD19, a protein expressed by normal B cells as well as B-cell leukemias and lymphomas, by investigators at the NCI, University of Pennsylvania, and Memorial Sloan Kettering Cancer Center demonstrated the clinical efficacy of CAR T cell therapies and resulted in complete remissions in many heavily pre-treated patients.
[15] These trials ultimately led in the US to the FDA's first two approvals of CAR T cells in 2017, those for tisagenlecleucel (Kymriah), marketed by Novartis originally for B-cell precursor acute lymphoblastic leukemia (B-ALL), and axicabtagene ciloleucel (Yescarta), marketed by Kite Pharma originally for diffuse large B-cell lymphoma (DLBCL).
The first approved treatments use CARs that target the antigen CD19, present in B-cell-derived cancers such as acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL).
[31][32] There are also efforts underway to engineer CARs targeting many other blood cancer antigens, including CD30 in refractory Hodgkin's lymphoma; CD33, CD123, and FLT3 in acute myeloid leukemia (AML); and BCMA in multiple myeloma.
[45] A regulatory T cell outfitted with a CAR could have the potential to confer tolerance to a specific antigen, something that could be utilized in organ transplantation or rheumatologic diseases like lupus.
EMA:005095, Label (Aucatzyl) There are serious side effects that result from CAR T-cells being introduced into the body, including cytokine release syndrome and neurological toxicity.
[76] Anaphylaxis may be a side effect, as the CAR is made with a foreign monoclonal antibody, and as a result provokes an immune response.
This results in the CAR T-cells attacking non-tumor tissue, such as healthy B cells that express CD19 causing B-cell aplasia.
The clinical manifestation of this syndrome resembles sepsis with high fever, fatigue, myalgia, nausea, capillary leakages, tachycardia and other cardiac dysfunction, liver failure, and kidney impairment.
[80] Early intervention using tocilizumab was shown to reduce the frequency of severe CRS in multiple studies[81][82] without affecting the therapeutic effect of the treatment.
A novel strategy aimed to ameliorate CRS is based on the simultaneous expression of an artificial non-signaling IL-6 receptor on the surface of CAR T-cells.
[83] This construct neutralizes macrophage-derived IL-6 through sequestration, thus decreasing the severity of CRS without interfering with the antitumor capability of the CAR T-cell itself.
[85] In another clinical trial sponsored by the Fred Hutchinson Cancer Research Center, there was one reported case of irreversible and fatal neurological toxicity 122 days after the administration of CAR T-cells.
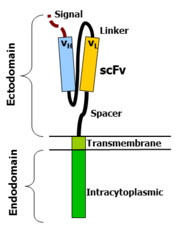
[89] An scFv is a chimeric protein made up of the light (VL) and heavy (VH) chains of immunoglobins, connected with a short linker peptide.
[92][42][93][94][95] In addition to antibody fragments, non-antibody-based approaches have also been used to direct CAR specificity, usually taking advantage of ligand/receptor pairs that normally bind to each other.
[88] The hinge, also called a spacer, is a small structural domain that sits between the antigen recognition region and the cell's outer membrane.
An ideal hinge enhances the flexibility of the scFv receptor head, reducing the spatial constraints between the CAR and its target antigen.
It anchors the CAR to the plasma membrane, bridging the extracellular hinge and antigen recognition domains with the intracellular signaling region.
The involvement of these intracellular signaling domains improve T cell proliferation, cytokine secretion, resistance to apoptosis, and in vivo persistence.
Herpes simplex virus thymidine kinase (HSV-TK) and inducible caspase 9 (iCasp9) are two types of suicide genes that have been integrated into CAR T cells.
Dosing the patient with rimiducid activates the suicide system, leading to rapid apoptosis of the genetically modified T cells.
Although both the HSV-TK and iCasp9 systems demonstrate a noticeable function as a safety switch in clinical trials, some defects limit their application.
[111] An in vivo study in mice shows that dual-receptor CAR T cells effectively eradicated prostate cancer and achieved complete long-term survival.
[112] ON-switch and OFF-switch: In this system, CAR T cells can only function in the presence of both tumor antigen and a benign exogenous molecule.
To achieve this, the CAR T cell's engineered chimeric antigen receptor is split into two separate proteins that must come together in order to function.
In the presence of an exogenous molecule (such as a rapamycin analog), the binding and signaling proteins dimerize together, allowing the CAR T cells to attack the tumor.
[122] Recent advancements in CAR T-cell therapy have focused on alternative activating domains to enhance efficacy and overcome resistance in solid tumors.
These strategies, including the use of nonconventional costimulatory molecules like MyD88/CD40,[131][132] highlight the innovative approaches being taken to optimize CAR T-cell therapies for more effective cancer treatments.

1. T cells are isolated from a patient's blood
2. A new gene encoding a chimeric antigen receptor is incorporated into the T cells
3. Engineered T cells are now specific to a desired target antigen
4. Engineered T cells are expanded in tissue culture
5. Engineered T cells are infused back into the patient