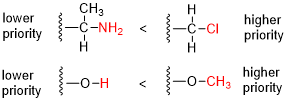Cahn–Ingold–Prelog priority rules
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules (also the CIP priority convention; named after Robert Sidney Cahn, Christopher Kelk Ingold, and Vladimir Prelog) are a standard process to completely and unequivocally name a stereoisomer of a molecule.
A molecule with an integer n describing the number of stereocenters will usually have 2n stereoisomers, and 2n−1 diastereomers each having an associated pair of enantiomers.
[3][4] The CIP sequence rules contribute to the precise naming of every stereoisomer of every organic molecule with all atoms of ligancy of fewer than 4 (but including ligancy of 6 as well, this term referring to the "number of neighboring atoms" bonded to a center).
[2]: 26ff The rules have since been revised, most recently in 2013,[7] as part of the IUPAC book Nomenclature of Organic Chemistry.
[11] When dealing with double bonded priority groups, one is allowed to visit the same atom twice as one creates an arc.
[12] When B is replaced with a list of attached atoms, A itself, but not its "phantom", is excluded in accordance with the general principle of not doubling back along a bond that has just been followed.
A stereoisomer that contains two higher priority groups on the same face of the double bond (cis) is classified as "Z."
The stereoisomer with two higher priority groups on opposite sides of a carbon-carbon double bond (trans) is classified as "E."[13] To handle a molecule containing one or more cycles, one must first expand it into a tree (called a hierarchical digraph) by traversing bonds in all possible paths starting at the stereocenter.
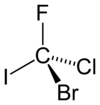
In a configurational isomer, the lowest priority group (most times hydrogen) is positioned behind the plane or the hatched bond going away from the reader.
[12] A practical method of determining whether an enantiomer is R or S is by using the right-hand rule: one wraps the molecule with the fingers in the direction 1 → 2 → 3.
[20] If a compound has more than one chiral stereocenter, each center is denoted by either R or S. For example, ephedrine exists in (1R,2S) and (1S,2R) stereoisomers, which are distinct mirror-image forms of each other, making them enantiomers.
A meso compound is superposable on its mirror image, therefore it reduces the number of stereoisomers predicted by the 2n rule.
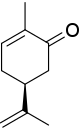
To begin, the lowest-numbered (according to IUPAC systematic numbering) stereogenic center is given the R* descriptor.
When the nucleophile attacks butanone, the faces are not identical (enantiotopic) and a racemic product results.
The same rules that determine the stereochemistry of a stereocenter (R or S) also apply when assigning the face of a molecular group.
Hydride addition as in a reduction process from this side will form the (S)-enantiomer and attack from the opposite Si-face will give the (R)-enantiomer.