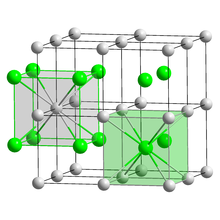
Caesium bromide
420 g/L (11 °C) See References 560 /L (15°C)1020 g/L (28.5 °C)1180 g/L (31 °C)1240 g/L (32.5 °C)1380 g/L (35 °C) Caesium bromide or cesium bromide is an ionic compound of caesium and bromine with the chemical formula CsBr.
It is a white or transparent solid with melting point at 636 °C that readily dissolves in water.
Its bulk crystals have the cubic CsCl structure, but the structure changes to the rocksalt type in nanometer-thin film grown on mica, LiF, KBr or NaCl substrates.
Caesium bromide is sometimes used in optics as a beamsplitter component in wide-band spectrophotometers.
* Crystran Ltd experimental data July 2021 Archived 2012-12-18 at the Wayback Machine