Caesium hexafluorocuprate(IV)
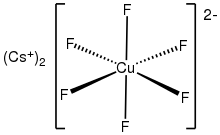
Caesium hexafluorocuprate is the inorganic compound with the chemical formula Cs2CuF6.
It is a red solid that degrades upon contact with water.
It was first prepared be heating CsCuCl3 and caesium fluoride at 410°C under 350 atmospheres of fluorine:[2] The anion [CuF6]2- is a rare example of a copper(IV) complex.
In terms of its electronic structure, the anion has a low-spin d7 configuration.
It is thus susceptible to Jahn-Teller distortion.