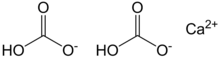
Calcium bicarbonate
The relative concentrations of these carbon-containing species depend on the pH; bicarbonate predominates within the range 6.36–10.25 in fresh water.
All waters in contact with the atmosphere absorb carbon dioxide, and as these waters come into contact with rocks and sediments they acquire metal ions, most commonly calcium and magnesium, so most natural waters that come from streams, lakes, and especially wells, can be regarded as dilute solutions of these bicarbonates.
As water containing carbon dioxide (including extra CO2 acquired from soil organisms) passes through limestone or other calcium carbonate-containing minerals, it dissolves part of the calcium carbonate, hence becomes richer in bicarbonate.
In the reverse process, dissolved carbon dioxide (CO2) in rainwater (H2O) reacts with limestone calcium carbonate (CaCO3) to form soluble calcium bicarbonate (Ca(HCO3)2).
In medicine, calcium bicarbonate is sometimes administered intravenously to immediately correct the cardiac depressor effects of hyperkalemia by increasing calcium concentration in serum, and at the same time, correcting the acid usually present.