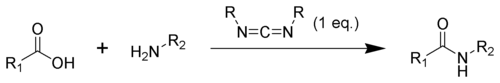
Carbodiimide
In organic chemistry, a carbodiimide (systematic IUPAC name: methanediimine[1]) is a functional group with the formula RN=C=NR.
On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its maser emissions.
[4] Solid diaryl carbodiimides are more stable, but can slowly undergo hydrolysis in the presence of water over time.
A typical reagent for this process is mercuric oxide:[9] This reaction can often be conducted as stated, even though carbodiimides react with water.
[11][12] Isocyanates can be converted to carbodiimides with loss of carbon dioxide:[13][4] The reaction is catalyzed by phosphine oxides.
[9] Compared to other heteroallenes, carbodiimides are very weak electrophiles and only react with nucleophiles in the presence of catalysts, such as acids.
Here the polycarbodiimide reacts with carboxylic acids, whose functional groups are often present in such aqueous resins, to form N-acyl urea.
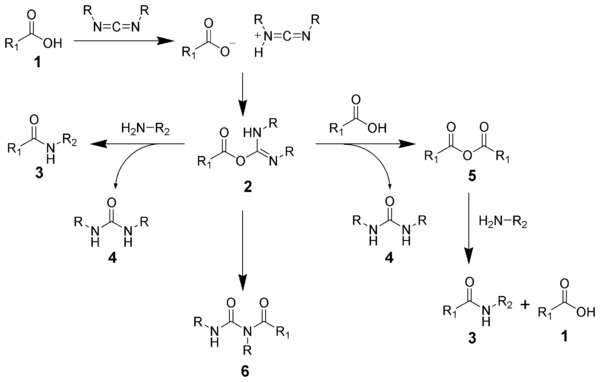
The acid 1 will react with the carbodiimide to produce the key intermediate: the O-acylisourea 2, which can be viewed as a carboxylic ester with an activated leaving group.
DCC has achieved popularity mainly because of its high-yielding amide coupling reactions and the fact that it is quite inexpensive.