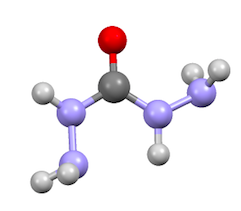
Carbohydrazide
It appears as a white solid that is soluble in water,[1][2] but not in many organic solvents, such as ethanol, ether or benzene.
They occur widely in the drugs, herbicides, plant growth regulators, and dyestuffs.
[2] It can be prepared from phosgene, but this route cogenerates the hydrazinium salt [N2H5]Cl and results in some diformylation.
All nitrogen centers are at least somewhat pyramidal, indicative of weaker C-N pi-bonding.
Carbohydrazide is harmful if swallowed, irritating to eyes, respiratory system, and skin.