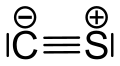Carbon monosulfide
Carbon monosulfide is a chemical compound with the formula CS.
This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interstellar medium.
The molecule is not intrinsically unstable, but it tends to polymerize in sunlight to a brown mass, as first discovered in 1868 and 1872.
This tendency towards polymerization reflects the greater stability of C–S single bonds.
Polymers with the formula (CS)n have been reported,[3] and the formal dimer is ethenedithione.