Caesium
The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy.
[13] In medieval and early modern writings caesius was spelled with the ligature æ as cæsius; hence, an alternative but now old-fashioned orthography is cæsium.
The others are rubidium (39 °C [102 °F]), francium (estimated at 27 °C [81 °F]), mercury (−39 °C [−38 °F]), and gallium (30 °C [86 °F]); bromine is also liquid at room temperature (melting at −7.2 °C [19.0 °F]), but it is a halogen and not a metal.
[22] The golden colour of caesium comes from the decreasing frequency of light required to excite electrons of the alkali metals as the group is descended.
Thus caesium transmits and partially absorbs violet light preferentially while other colours (having lower frequency) are reflected; hence it appears yellowish.
[35] CsOH has been previously regarded by chemists as the "strongest base", reflecting the relatively weak attraction between the large Cs+ ion and OH−;[24] it is indeed the strongest Arrhenius base; however, a number of compounds such as n-butyllithium, sodium amide, sodium hydride, caesium hydride, etc., which cannot be dissolved in water as reacting violently with it but rather only used in some anhydrous polar aprotic solvents, are far more basic on the basis of the Brønsted–Lowry acid–base theory.
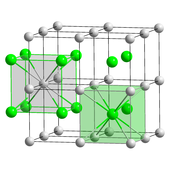
Also called the "caesium chloride structure",[31] this structural motif is composed of a primitive cubic lattice with a two-atom basis, each with an eightfold coordination; the chloride atoms lie upon the lattice points at the edges of the cube, while the caesium atoms lie in the holes in the centre of the cubes.
Several of these are synthesized from lighter elements by the slow neutron capture process (S-process) inside old stars[51] and by the R-process in supernova explosions.
[56] However, this fission product yield is reduced in most reactors because the predecessor, 135Xe, is a potent neutron poison and frequently transmutes to stable 136Xe before it can decay to 135Cs.
[57][58] The beta decay from 137Cs to 137mBa results in gamma radiation as the 137mBa relaxes to ground state 137Ba, with the emitted photons having an energy of 0.6617 MeV.
[63] Because iodine and xenon are volatile and can diffuse through nuclear fuel or air, radioactive caesium is often created far from the original site of fission.
To supply the developing market, Cabot Corporation built a production plant in 1997 at the Tanco mine near Bernic Lake in Manitoba, with a capacity of 12,000 barrels (1,900 m3) per year of caesium formate solution.
Because of the bright blue lines in the emission spectrum, they derived the name from the Latin word caesius, meaning 'bluish grey'.
[75] The pure metal was eventually isolated by the Swedish chemist Carl Setterberg while working on his doctorate with Kekulé and Bunsen.
Very few applications existed for caesium until the 1920s, when it came into use in radio vacuum tubes, where it had two functions; as a getter, it removed excess oxygen after manufacture, and as a coating on the heated cathode, it increased the electrical conductivity.
[79] Applications for nonradioactive caesium included photoelectric cells, photomultiplier tubes, optical components of infrared spectrophotometers, catalysts for several organic reactions, crystals for scintillation counters, and in magnetohydrodynamic power generators.
The International System of Units (SI) defines the second as the duration of 9,192,631,770 cycles at the microwave frequency of the spectral line corresponding to the transition between two hyperfine energy levels of the ground state of caesium-133.
[80] The 13th General Conference on Weights and Measures of 1967 defined a second as: "the duration of 9,192,631,770 cycles of microwave light absorbed or emitted by the hyperfine transition of caesium-133 atoms in their ground state undisturbed by external fields".
"[80] These clocks measure frequency with an error of 2 to 3 parts in 1014, which corresponds to an accuracy of 2 nanoseconds per day, or one second in 1.4 million years.
In the two-electrode vacuum tube converter, caesium neutralizes the space charge near the cathode and enhances the current flow.
[94] This technology is used primarily in the isolation of viral particles, subcellular organelles and fractions, and nucleic acids from biological samples.
[96] Doping with caesium compounds enhances the effectiveness of several metal-ion catalysts for chemical synthesis, such as acrylic acid, anthraquinone, ethylene oxide, methanol, phthalic anhydride, styrene, methyl methacrylate monomers, and various olefins.
Its advantages include a half-life of roughly 30 years, its availability from the nuclear fuel cycle, and having 137Ba as a stable end product.
The high water solubility is a disadvantage which makes it incompatible with large pool irradiators for food and medical supplies.
[105] Caesium and mercury were used as a propellant in early ion engines designed for spacecraft propulsion on very long interplanetary or extraplanetary missions.
[111] Caesium compounds may have been used as fuel additives to reduce the radar signature of exhaust plumes in the Lockheed A-12 CIA reconnaissance aircraft.
[112] Caesium and rubidium have been added as a carbonate to glass because they reduce electrical conductivity and improve stability and durability of fibre optics and night vision devices.
The hydrogen gas produced by the reaction is heated by the thermal energy released at the same time, causing ignition and a violent explosion.
[126][127] Experiments with dogs showed that a single dose of 3.8 millicuries (140 MBq, 4.1 μg of caesium-137) per kilogram is lethal within three weeks;[128] smaller amounts may cause infertility and cancer.
[129] The International Atomic Energy Agency and other sources have warned that radioactive materials, such as caesium-137, could be used in radiological dispersion devices, or "dirty bombs".




11 O
3 cluster







