Chan–Lam coupling
Dominic Chan, David Evans, and Patrick Lam published their work nearly simultaneously.
Later developments by others extended the scope to include using carboxylic acids, giving aryl-ester products.
[7] Analysis of the mechanism is complicated by the lability of copper reagents and the multicomponent nature of the reaction.
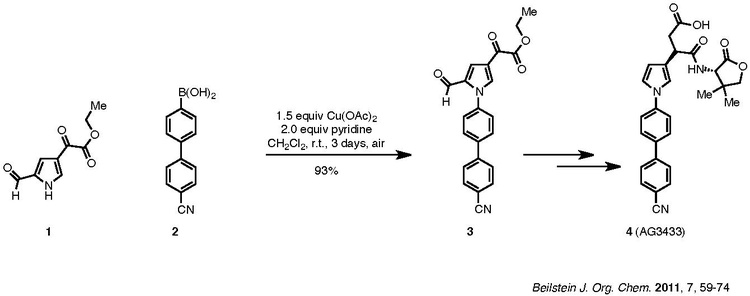
A copper(III)-aryl-alkoxide or copper(III)-aryl-amide intermediate undergoes Reductive elimination to give the aryl ether or aryl amine, respectively: An example of the Chan–Lam coupling to synthesize biologically active compounds is shown below: Compound 1, a pyrrole, is coupled with aryl boronic acid, 2, to afford product 3, which is then carried forward to the target 4.
Although the reaction requires three days, it was carried out at room temperature in ambient air and resulted in a 93% yield.