Reductive elimination
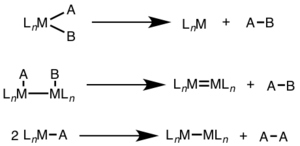
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands.
Since oxidative addition and reductive elimination are reverse reactions, the same mechanisms apply for both processes, and the product equilibrium depends on the thermodynamics of both directions.
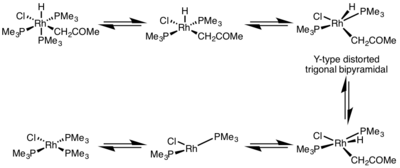
This complex adopts a Y-type distorted trigonal bipyramidal structure where a π-donor ligand is at the basal position and the two groups to be eliminated are brought very close together.
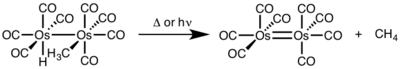
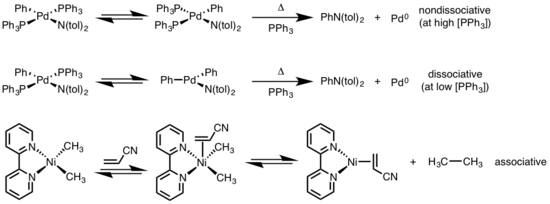
[6][7] Reductive elimination is sensitive to a variety of factors including: (1) metal identity and electron density, (2) sterics, (3) participating ligands, (4) coordination number, (5) geometry, and (6) photolysis/oxidation.
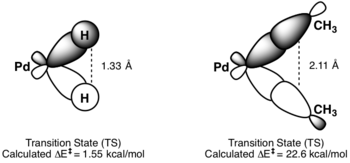
[9] Kinetics for reductive elimination are hard to predict, but reactions that involve hydrides are particularly fast due to effects of orbital overlap in the transition state.
When reductive elimination occurs from odd coordination number complexes, the resulting intermediate occupies a nonbonding molecular orbital.
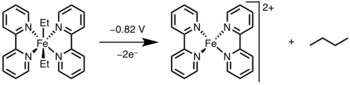
[12] Reductive elimination has found widespread application in academia and industry, most notable being hydrogenation,[13] the Monsanto acetic acid process,[14] hydroformylation,[15] and cross-coupling reactions.