Chaperone (protein)
The specific mode of function of chaperones differs based on their target proteins and location.
[4] Heat shock protein chaperones are classified based on their observed molecular weights into Hsp60, Hsp70, Hsp90, Hsp104, and small Hsps.
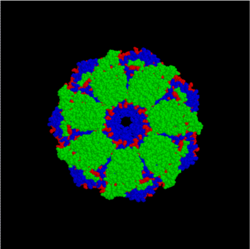
[5] The Hsp60 family of protein chaperones are termed chaperonins, and are characterized by a stacked double-ring structure and are found in prokaryotes, in the cytosol of eukaryotes, and in mitochondria.
Although most newly synthesized proteins can fold in absence of chaperones, a minority strictly requires them for the same.
[6] Chaperones can also work as disaggregases, which interact with aberrant protein assemblies and revert them to monomers.
[10][11] Crowding may also increase the effectiveness of the chaperone proteins such as GroEL,[12] which could counteract this reduction in folding efficiency.
[13] Some highly specific 'steric chaperones' convey unique structural information onto proteins, which cannot be folded spontaneously.
A bacterial translocation-specific chaperone SecB maintains newly synthesized precursor polypeptide chains in a translocation-competent (generally unfolded) state and guides them to the translocon.
Chaperones are found extensively in the endoplasmic reticulum (ER), since protein synthesis often occurs in this area.
In the endoplasmic reticulum (ER) there are general, lectin- and non-classical molecular chaperones that moderate protein folding.
GroEL (Hsp60) is a double-ring 14mer with a hydrophobic patch at its opening; it is so large it can accommodate native folding of 54-kDa GFP in its lumen.
[27] It has been noted that increased expression of Hsp70 proteins in the cell results in a decreased tendency toward apoptosis.
Proteins in the Hsp100/Clp family form large hexameric structures with unfoldase activity in the presence of ATP.
[33] Most of these proteins proved to be either major or minor structural components of the completed phage particle.
[36][35] Gp4(50)(65), although not specifically listed as a chaperone, acts catalytically as a nuclease that appears to be essential for morphogenesis by cleaving packaged DNA to enable the joining of heads to tails.
[44] There are many disorders associated with mutations in genes encoding chaperones (i.e. multisystem proteinopathy) that can affect muscle, bone and/or the central nervous system.