Chemotaxis
[4] The aberrant chemotaxis of leukocytes and lymphocytes also contribute to inflammatory diseases such as atherosclerosis, asthma, and arthritis.
[10] The Nobel Prize laureate I. Metchnikoff also contributed to the study of the field during 1882 to 1886, with investigations of the process as an initial step of phagocytosis.
[11] The significance of chemotaxis in biology and clinical pathology was widely accepted in the 1930s, and the most fundamental definitions underlying the phenomenon were drafted by this time.
The pioneering works of J. Adler modernized Pfeffer's capillary assay and represented a significant turning point in understanding the whole process of intracellular signal transduction of bacteria.
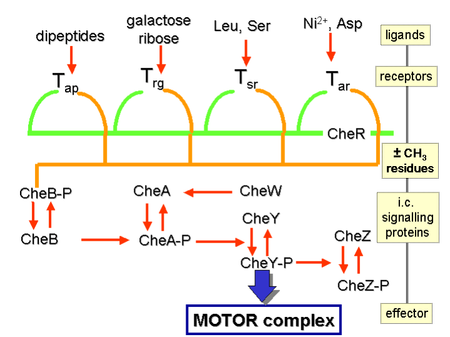
[25][page needed] Chemical gradients are sensed through multiple transmembrane receptors, called methyl-accepting chemotaxis proteins (MCPs), which vary in the molecules that they detect.
Change in the rotation state of a single flagellum can disrupt the entire flagella bundle and cause a tumble.
[citation needed] This regulation allows the bacterium to 'remember' chemical concentrations from the recent past, a few seconds, and compare them to those it is currently experiencing, thus 'know' whether it is traveling up or down a gradient.
[36] Chemoattractants or chemorepellents bind MCPs at its extracellular domain; an intracellular signaling domain relays the changes in concentration of these chemotactic ligands to downstream proteins like that of CheA which then relays this signal to flagellar motors via phosphorylated CheY (CheY-P).
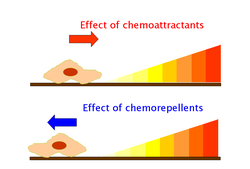
Formyl peptides, such as fMLF, attract leukocytes such as neutrophils and macrophages, causing movement toward infection sites.
[39] The mechanism of chemotaxis that eukaryotic cells employ is quite different from that in the bacteria E. coli; however, sensing of chemical gradients is still a crucial step in the process.
[40][better source needed] Due to their small size and other biophysical constraints, E. coli cannot directly detect a concentration gradient.
[41] Instead, they employ temporal gradient sensing, where they move over larger distances several times their own width and measure the rate at which perceived chemical concentration changes.
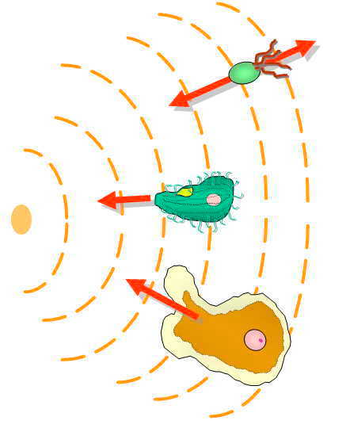
[42] Eukaryotic chemotaxis involves detecting a concentration gradient spatially by comparing the asymmetric activation of these receptors at the different ends of the cell.
[42] In mating yeast, which are non-motile, patches of polarity proteins on the cell cortex can relocate in a chemotactic fashion up pheromone gradients.
[43][45] In prokaryotes, this mechanism involves the methylation of receptors called methyl-accepting chemotaxis proteins (MCPs).
[43] In contrast, chemotactic memory in eukaryotes can be explained by the Local Excitation Global Inhibition (LEGI) model.
[45][46] LEGI involves the balance between a fast excitation and delayed inhibition which controls downstream signaling such as Ras activation and PIP3 production.
[47] Levels of receptors, intracellular signalling pathways and the effector mechanisms all represent diverse, eukaryotic-type components.
[50] Chemotaxis has high significance in the early phases of embryogenesis as development of germ layers is guided by gradients of signal molecules.
The growing distal end of actin filaments develops connections with the internal surface of the plasma membrane via different sets of peptides and results in the formation of anterior pseudopods and posterior uropods.
In general, eukaryotic cells sense the presence of chemotactic stimuli through the use of 7-transmembrane (or serpentine) heterotrimeric G-protein-coupled receptors, a class representing a significant portion of the genome.
[62] While some chemotaxis receptors are expressed in the surface membrane with long-term characteristics, as they are determined genetically, others have short-term dynamics, as they are assembled ad hoc in the presence of the ligand.
Altered chemotactic activity of extracellular (e.g., Escherichia coli) or intracellular (e.g., Listeria monocytogenes) pathogens itself represents a significant clinical target.
Modification of endogenous chemotactic ability of these microorganisms by pharmaceutical agents can decrease or inhibit the ratio of infections or spreading of infectious diseases.
Apart from infections, there are some other diseases wherein impaired chemotaxis is the primary etiological factor, as in Chédiak–Higashi syndrome, where giant intracellular vesicles inhibit normal migration of cells.
Although interactions of the factors listed above make the behavior of the solutions of mathematical models of chemotaxis rather complex, it is possible to describe the basic phenomenon of chemotaxis-driven motion in a straightforward way.
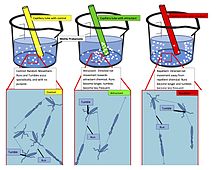
A wide range of techniques is available to evaluate chemotactic activity of cells or the chemoattractant and chemorepellent character of ligands.
The basic requirements of the measurement are as follows: Despite the fact that an ideal chemotaxis assay is still not available, there are several protocols and pieces of equipment that offer good correspondence with the conditions described above.
[78] The thermodynamically favorable binding of enzymes to their specific substrates is recognized as the origin of enzymatic chemotaxis.
This has been demonstrated by using dye molecules that move directionally in gradients of polymer solution through favorable hydrophobic interactions.