Chlorine production
Chlorine gas can be produced by extracting from natural materials, including the electrolysis of a sodium chloride solution (brine) and other ways.
The production of chlorine results in the co-products caustic soda (sodium hydroxide, NaOH) and hydrogen gas (H2).
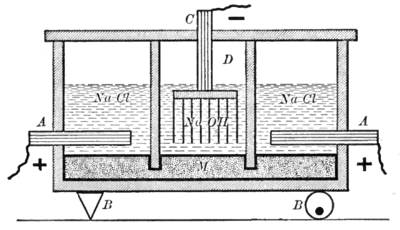
There are three industrial methods for the extraction of chlorine by electrolysis of chloride solutions, all proceeding according to the following equations: Overall process: 2 NaCl (or KCl) + 2 H2O → Cl2 + H2 + 2 NaOH (or KOH) Mercury cell electrolysis, also known as the Castner–Kellner process, was the first method used at the end of the nineteenth century to produce chlorine on an industrial scale.
When a potential difference is applied and current flows, chlorine is released at the titanium anode and sodium (or potassium) dissolves in the mercury cathode forming an amalgam.
This flows continuously into a separate reactor ("denuder" or "secondary cell"), where it is usually converted back to mercury by reaction with water, producing hydrogen and sodium (or potassium) hydroxide at a commercially useful concentration (50% by weight).
In Japan, mercury-based chloralkali production was virtually phased out by 1987 (except for the last two potassium chloride units shut down in 2003).
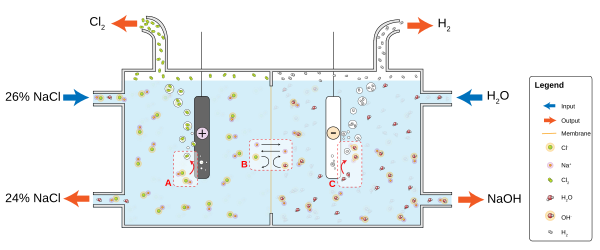
Diaphragm cells are not burdened with the problem of preventing mercury discharge into the environment; they also operate at a lower voltage, resulting in an energy savings over the mercury cell method,[8] but large amounts of steam are required if the caustic has to be evaporated to the commercial concentration of 50%.
Saturated sodium (or potassium) chloride solution is passed through the anode compartment, leaving at a lower concentration.
A portion of the concentrated sodium hydroxide solution leaving the cell is diverted as product, while the remainder is diluted with deionized water and passed through the electrolysis apparatus again.
Although a much lower production scale is involved, electrolytic diaphragm and membrane technologies are also used industrially to recover chlorine from hydrochloric acid solutions, producing hydrogen (but no caustic alkali) as a co-product.
Furthermore, electrolysis of fused chloride salts (Downs process) also enables chlorine to be produced, in this case as a by-product of the manufacture of metallic sodium or magnesium.
Due to the extremely corrosive reaction mixture, industrial use of this method is difficult and several pilot trials failed in the past.
[11] Small amounts of chlorine gas can be made in the laboratory by putting concentrated hydrochloric acid in a flask with a side arm and rubber tubing attached.
The reactions are often carried out in a series of reactors before the treated brine is sent to a large clarifier where the calcium carbonate and magnesium hydroxide are settled out.
After the ion exchangers, the brine is considered pure, and is transferred to storage tanks to be pumped into the cell room.
Brine exiting the cell room must be treated to remove residual chlorine and control pH levels before being returned to the saturation stage.
After cooling the gas stream passes through a series of towers with counter flowing sulfuric acid.
Non condensible gases and remaining chlorine gas are vented off as part of the pressure control of the liquefaction systems.
Hydrogen produced as a byproduct may be vented unprocessed directly to the atmosphere or cooled, compressed and dried for use in other processes on site or sold to a customer via pipeline, cylinders or trucks.
[15] Since electricity is an indispensable raw material for the production of chlorine, the energy consumption corresponding to the electrochemical reaction cannot be reduced.
If hydropower, nuclear power or other low carbon sources are used, emissions will be much lower than if fossil fuels are used.