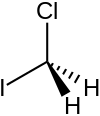
Chloroiodomethane
[2] Chloroiodomethane is used in cyclopropanation (Simmon-Smith reaction), where it often replaces diiodomethane because of higher yields and selectivity.
It is also used in Mannich reaction, aminomethylation, epoxidation, ring opening and addition to terminal alkenes.
It is a precursor to agent Ph3P=CHCl that can add a chloromethylene group (=CHCl).
[1] It reacts with organolithium compounds to give chloromethyl lithium (ClCH2Li).
[3] It crystallizes orthorhombic crystal system with space group Pnma with lattice constants: a = 6.383, b = 6.706, c = 8.867 (.10−1 nm).