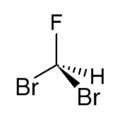
Dibromofluoromethane
[1] It is soluble in alcohol, acetone, benzene and chloroform.
It is prepared from dibromomethane and antimony(III) fluoride.
[2] It can be used to prepare bromofluoromethane by reductive debromination with organotin hydride as tributyltin hydride.
[3] Its ozone depletion potential (ODP) is 1.0 and it is included in list of Class I Ozone-Depleting Substances.
This article about an organic halide is a stub.