Cholestasis
[27] Typical symptoms of DIC include pruritus and jaundice, nausea, fatigue, and dark urine, which usually resolve after discontinuation of the offending medication.
Other symptoms include pruritus and skin lesions, and in prolonged cholestasis, malabsorption and steatorrhea leading to fat-soluble vitamin deficiency.
Clinically, diagnosis generally requires a 1:40 or greater titer of anti-mitochondrial antibody (AMA) against PDC-E2 and elevated alkaline phosphatase persisting for 6+ months.
A loss of immune tolerance is indicated by the presence of AMAs and autoreactive CD4+ and CD8+ T cells targeting cholangiocytes that line the bile ducts.
[34] Genetic predisposition is suggested by high concordance between identical twins, higher incidence among relatives, and a strong association of disease with certain HLA variants.
[34] Disease is likely triggered in the genetically predisposed by some environmental factor, such as pollutants, xenobiotics (e.g., chemicals in makeup), diet, drugs, stress, and infectious agents.
[41] Though 40-50% of patients are asymptomatic, commonly reported symptoms include abdominal pain in the right upper quadrant, pruritus, jaundice, fatigue, and fever.
[40] Prolonged cholestasis in PSC may cause fat-soluble vitamin deficiency leading to osteoporosis[40] Diagnosis requires elevated serum alkaline phosphatase persisting for at least 6 months and the presence of bile duct strictures on cholangiogram.
[40] Although the pathogenesis of PSC is poorly understood, three dominant theories have been proposed: 1) aberrant immune response, 2) increased intestinal permeability, and 3) dysbiosis of gut microbiota.
[40] Further evidence for genetic predisposition include the identification of 23 non-HLA susceptibility loci and a higher disease risk among siblings, though environmental factors appear to play a much greater role in pathogenesis.
[44] Leaky tight junctions could allow commensal bacteria and toxins to enter portal circulation and reach the liver, where they can trigger inflammation and fibrosis.
[44] The intestinal dysbiosis theory hypothesizes that yet unidentified environmental triggers (e.g., diet, medication, inflammation) reduce microbiota diversity and/or alter the population of specific species.
[45][42] DCA and LCA are then reabsorbed into portal circulation and reach the liver, where they serve as signaling molecules that maintain bile acid homeostasis.
[48] Diagnosis usually occurs by analyzing laboratory features, liver biopsy results, DNA/RNA sequences, and biliary lipid analysis.
[52] Alagille syndrome is an autosomal dominant disorder that impacts five systems, including the liver, heart, skeleton, face, and eyes.
[48] Without enteral food consumption, gallbladder function is greatly inhibited, leading to gallstone formation, subsequent blockage, and eventually cholestasis.
[59] Intravenous glucose can also cause cholestasis as a result of increased fatty acid synthesis and decreased breakdown, which facilitates the accumulation of fats.
Drugs may induce cholestasis by interfering with 1) hepatic transporters, 2) bile canaliculi dynamics, and/or 3) cell structure and protein localization.
[24][66] Cholestasis can result from competitive inhibition of BSEP by several drugs, including cyclosporine A, rifampicin, nefazodone, glibenclamide, troglitazone, and bosentan.
[75] Enterohepatic bile flow requires the concerted activity of both NTCP and BSEP, which form the major route by which BAs enter and exit hepatocytes respectively.
[66] Therefore, NTCP inhibitors, such as cyclosporine A, ketoconazole, propranolol, furosemide, rifamycin, saquinavir, and ritonavir, should theoretically cause cholestasis by decreasing hepatocyte BA uptake.
[75] However, no relationship was found between NTCP inhibition and DIC risk,[75] possibly because basolateral sodium-independent OATPs can partially compensate for bile salt uptake.
If a drug causes internalization of hepatocyte tight junctions, like rifampicin does in mice, bile flow may become impaired due to paracellular leakage.
[66] Membrane fluidity can affect bile flow by regulating the activity of hepatocyte Na+/K+-ATPase, which maintains the inwardly-directed Na+ gradient that drives BA uptake by apical NTCP.
[66] Lastly, some agents (rimpaficin and 17β-estradiol) were shown to hinder proper localization of hepatocyte transporters by interfering with the microtubules required for their insertion into plasma membranes.
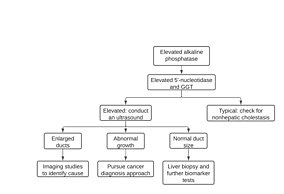
[80] However, an elevation that exceeds 10 times the upper baseline limit is strongly indicative of either intrahepatic or extrahepatic cholestasis and requires further investigation.
Back pressure created from obstructive cholestasis can cause dilation of the bile duct and biliary epithelial cell proliferation, mainly in the portal tracts.
However, with advancing technology in the molecular biochemistry field and higher understanding of bile acid regulation, novel pharmacological treatments have been considered.
[124] Obeticholic acid has been approved by the US Food and Drug Administration for PBC in 2016 after experiments found beneficial improvements for the liver in half of patients with inadequate response to UDCA.
[139] A recent scientific breakthrough for cholestasis that has allowed us to evaluate a new treatment option is that a hydrophilic environment and bicarbonate production protects hepatocytes from bile acid.