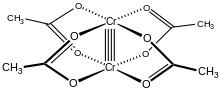
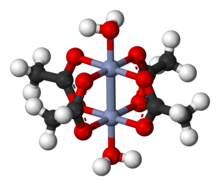
Chromium(II) acetate
The preparation of chromous acetate once was a standard test of the synthetic skills of students due to its sensitivity to air and the dramatic colour changes that accompany its oxidation.
Consistent with the fact that it is nonionic, Cr2(OAc)4(H2O)2 exhibits poor solubility in water and methanol.
This quadruple bond is also confirmed by the low magnetic moment and short intermolecular distance between the two atoms of 236.2 ± 0.1 pm.
[5] The preparation usually begins with reduction of an aqueous solution of a Cr(III) compound using zinc.
The synthesis of Cr2(OAc)4(H2O)2 has been traditionally used to test the synthetic skills and patience of inorganic laboratory students in universities because the accidental introduction of a small amount of air into the apparatus is readily indicated by the discoloration of the otherwise bright red product.