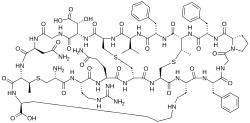
Cinnamycin
Based on NMR experiments, the binding pocket of cinnamycin consists of 7-14 amino acid residues which can accommodate the substrate phosphatidylethanolamine (PE).
The LanM family proteins encoded by the cinM gene are responsible for the dehydration of serine and threonine residues in the propeptide followed by the subsequent formation of lanthionine bridges.
[7] Lantibiotics are a group of ribosomally synthesized, post-translationally modified antimicrobial peptides with characteristic lanthionine(Lan) and methyllanthionine (MeLan) thioether crosslinks.
The biosynthesis of cinnamycin is encoded by the cin biosynthetic gene cluster and the synthesis is initiated when the structural gene lanA encodes the precursor peptide which carries an N-terminal extension called "leader peptide" which is 59 amino acid residues long is recognized by various enzymes to process the C-terminal propeptide which is 19 amino acid residues long and will be transformed into cinnamycin through post-translational modifications.
A cinnamycin specific-protease is absent in the gene cluster and hence the sequence is recognized by type I signal peptidase of the general secretory (sec) pathway.
Based on the structure of cinnamycin-PE complex, monomethylated PE will not fit into the binding pocket of cinnamycin and the inhibitory action will no longer be supported.
Type A lantibiotics are elongated, flexible, rod like molecules that are positively charged and act on bacterial membranes by the formation of pores.
These compounds are all derived from 19-aa propeptides and have one Lan, two MeLan and an unusual lysinoalanine bridge between Lys-19 and Ser-6 and an erythro-3-hydroxy-L-aspartic acid at position 15 which mediates the interaction between cinnamycin and its biological target phosphatidylethanolamine and hence important for their antimicrobial activity.
[7] In addition to the antimicrobial properties, cinnamycin-like peptides exhibit inhibitory actions against the angiotensin-converting enzyme, the activity of phospholipase A2, proliferation of herpes simplex virus, prostaglandin, and leucotriene biosynthesis.
These molecules consist of a well-defined pocket set up by the four cyclization events and recognizes phosphatidylethanolamine (PE) with a high affinity and selectivity.
[3] The compounds like duramycin and cinnamycin which disrupt PE association with phosphatidylserine receptors necessary for entry of many enveloped viruses is a promising strategy for broad-spectrum antiviral activity.