Claus process
[1] Hydrogen sulfide produced, for example, in the hydro-desulfurization of refinery naphthas and other petroleum oils, is converted to sulfur in Claus plants.
[6] Claus was born in Kassel in the German State of Hesse in 1827, and studied chemistry in Marburg before he emigrated to England in 1852.
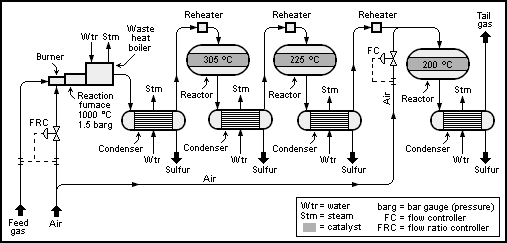
The H2S content and the concentration of other combustible components (hydrocarbons or ammonia) determine the location where the feed gas is burned.
[11] Gases containing ammonia, such as the gas from the refinery's sour water stripper (SWS), or hydrocarbons are converted in the burner muffle.
The separation of the combustion processes ensures an accurate dosage of the required air volume needed as a function of the feed gas composition.
To reduce the process gas volume or obtain higher combustion temperatures, the air requirement can also be covered by injecting pure oxygen.
Several technologies utilizing high-level and low-level oxygen enrichment are available in industry, which requires the use of a special burner in the reaction furnace for this process option.
The sulfur forms in the thermal phase as highly reactive S2 diradicals which combine exclusively to the S8 allotrope: Other chemical processes taking place in the thermal step of the Claus reaction are:[5] The Claus reaction continues in the catalytic step with activated aluminum(III) or titanium(IV) oxide, and serves to boost the sulfur yield.
One suggested mechanism is that S6 and S8 desorb from the catalyst's active sites with simultaneous formation of stable cyclic elemental sulfur.
Where an incineration or tail-gas treatment unit (TGTU) is added downstream of the Claus plant, only two catalytic stages are usually installed.
The high temperature in the first stage also helps to hydrolyze COS and CS2, which is formed in the furnace and would not otherwise be converted in the modified Claus process.
The catalytic conversion is maximized at lower temperatures, but care must be taken to ensure that each bed is operated above the dew point of sulfur.
The process has also to be applied to heavy petroleum extracted from oil sands deposits because sulfur accumulates in the heaviest fractions of hydrocarbons.