Hydrogen
Henry Cavendish, in 1766–81, identified hydrogen gas as a distinct substance[15] and discovered its property of producing water when burned; hence its name means "water-former" in Greek.
Hydrogen, typically nonmetallic except under extreme pressure, readily forms covalent bonds with most nonmetals, contributing to the formation of compounds like water and various organic substances.
Although tightly bonded to water molecules, protons strongly affect the behavior of aqueous solutions, as reflected in the importance of pH.
[23] The ground state energy level of the electron in a hydrogen atom is −13.6 eV,[24] equivalent to an ultraviolet photon of roughly 91 nm wavelength.
The hydrogen spectral series corresponds to emission of light due to transitions from higher to lower energy levels.
[34] Catalysts for the ortho-para interconversion, such as ferric oxide and activated carbon compounds, are used during hydrogen cooling to avoid this loss of liquid.
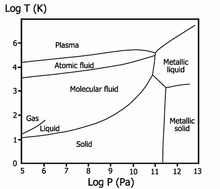
[38] Metallic hydrogen, a phase obtained at extremely high pressures (in excess of 400 gigapascals (3,900,000 atm; 58,000,000 psi)), is an electrical conductor.
[53] The exotic atom muonium (symbol Mu), composed of an antimuon and an electron, can also be considered a light radioisotope of hydrogen.
[62][63] Boyle did not note that the gas was inflammable, but hydrogen would play a key role in overturning the phlogiston theory of combustion.
He speculated that "inflammable air" was in fact identical to the hypothetical substance "phlogiston"[65][66] and further finding in 1781 that the gas produces water when burned.
According to quantum theory, this behavior arises from the spacing of the (quantized) rotational energy levels, which are particularly wide-spaced in H2 because of its low mass.
These widely spaced levels inhibit equal partition of heat energy into rotational motion in hydrogen at low temperatures.
[73] Hydrogen's unique position as the only neutral atom for which the Schrödinger equation can be directly solved, has significantly contributed to the understanding of quantum mechanics through the exploration of its energetics.
Regular passenger service resumed in the 1920s and the discovery of helium reserves in the United States promised increased safety, but the U.S. government refused to sell the gas for this purpose.
[citation needed] Deuterium was discovered in December 1931 by Harold Urey, and tritium was prepared in 1934 by Ernest Rutherford, Mark Oliphant, and Paul Harteck.
The alkali and alkaline earth metals, aluminium, zinc, manganese, and iron react readily with aqueous acids.
With heating, H2 reacts efficiently with the alkali and alkaline earth metals to give the saline hydrides of the formula MH and MH2, respectively.
The large amount of neutral hydrogen found in the damped Lyman-alpha systems is thought to dominate the cosmological baryonic density of the universe up to a redshift of z = 4.
[citation needed] Hydrogen is the third most abundant element on the Earth's surface,[103] mostly in the form of chemical compounds such as hydrocarbons and water.
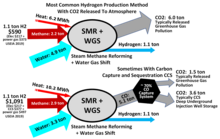
[107][108]: 1 Many methods exist for producing H2, but three dominate commercially: steam reforming often coupled to water-gas shift, partial oxidation of hydrocarbons, and water electrolysis.
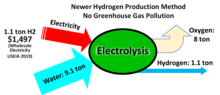
[117] The electrolysis process is more expensive than producing hydrogen from methane without carbon capture and storage and the efficiency of energy conversion is inherently low.
[118] Hydrogen produced in this manner could play a significant role in decarbonizing energy systems where there are challenges and limitations to replacing fossil fuels with direct use of electricity.
A few organisms, including the alga Chlamydomonas reinhardtii and cyanobacteria, have evolved a second step in the dark reactions in which protons and electrons are reduced to form H2 gas by specialized hydrogenases in the chloroplast.
[132] A number of labs (including in France, Germany, Greece, Japan, and the United States) are developing thermochemical methods to produce hydrogen from solar energy and water.
[149] These properties may be useful when hydrogen is purified by passage through hot palladium disks, but the gas's high solubility is also a metallurgical problem, contributing to the embrittlement of many metals,[150] complicating the design of pipelines and storage tanks.
The problem with this material is that after release of H2, the resulting boron nitride does not re-add H2, i.e. ammonia borane is an irreversible hydrogen carrier.
[152] More attractive, somewhat ironically, are hydrocarbons such as tetrahydroquinoline, which reversibly release some H2 when heated in the presence of a catalyst:[153] Large quantities of H2 are used in the "upgrading" of fossil fuels.
[156] Hydrogen is commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules.
[citation needed] Hydrogen (H2) is widely discussed as a carrier of energy with potential to help to decarbonize economies and mitigate greenhouse gas emissions.
[119] For example, in steelmaking, hydrogen could function as a clean energy carrier and also as a low-carbon catalyst, replacing coal-derived coke (carbon):[158] Hydrogen used to decarbonise transportation is likely to find its largest applications in shipping, aviation and, to a lesser extent, heavy goods vehicles, through the use of hydrogen-derived synthetic fuels such as ammonia and methanol and fuel cell technology.