Cobalt(II) bromide
In its anhydrous form, it is a green solid that is soluble in water, used primarily as a catalyst in some processes.
When anhydrous, cobalt(II) bromide appears as green crystals.
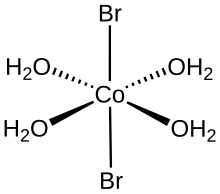
It is hygroscopic and eventually forms the hexahydrate in air,[1] which appears as red-purple crystals.
[4] Cobalt(II) bromide can be prepared as a hydrate by the reaction of cobalt hydroxide with hydrobromic acid: The classical coordination compound bromopentaamminecobalt(III) bromide is prepared by oxidation of an aqueous solution of cobalt(II) bromide and ammonia.
[5] Triphenylphosphine complexes of cobalt(II) bromide have been used as a catalysts in organic synthesis.