Cobalt(II) carbonate
This pink paramagnetic solid is an intermediate in the hydrometallurgical purification of cobalt from its ores.
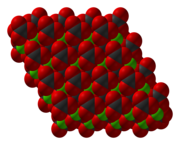
[5] CoCO3 adopts a structure like calcite, consisting of cobalt in an octahedral coordination geometry.
[8] Heating the carbonate proceeds in a typical way for calcining, except that the product becomes partially oxidized: The resulting Co3O4 converts reversibly to CoO at high temperatures.
[10] The moderately rare spherocobaltite is a natural form of cobalt carbonate, with good specimens coming especially from the Republic of Congo.
Animals, including humans, require trace amounts of cobalt, a component of vitamin B12.