Colored gold
The strength of gold-nickel-copper alloys is caused by formation of two phases: a gold-rich Au-Cu, and a nickel-rich Ni-Cu, and the resulting hardening of the material.
[3] The nickel used in some white gold alloys can cause an allergic reaction when worn over long periods (also notably on some wristwatch casings).
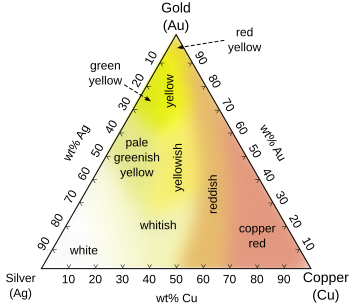
During ancient times, due to impurities in the smelting process, gold frequently turned a reddish color.
[citation needed] Some gold-copper-aluminium alloys form a fine surface texture at heat treatment, yielding a spangling effect.
A polished object is heated in hot oil to 150–200 °C for 10 minutes then cooled below 20 °C, forming a sparkly surface covered with tiny facets.
[4] However, electrum was used even thousands of years before that, by both the Akkadians and Ancient Egyptians (as evidenced by the Royal Cemetery at Ur).
[citation needed] A cheaper alternative which does not use palladium is made by adding silver, manganese, and copper to the gold in specific ratios.
Slightly nonstoichiometric compositions are used, however, to achieve a fine-grained two- or three-phase microstructure with reduced brittleness.
Purple gold is more brittle than other gold alloys (called the "purple plague" when it forms and causes serious faults in electronics[14]), as it is an intermetallic compound instead of a malleable alloy, and a sharp blow may cause it to shatter.
At a lower content of gold, the material is composed of the intermetallic and an aluminium-rich solid solution phase.
At a higher content of gold, the gold-richer intermetallic AuAl forms; the purple color is preserved to about 15% of aluminium.
A heat treatment then causes interdiffusion of the metals and formation of the required intermetallic compound.
[16][17] Black-colored gold can be produced by various methods: A range of colors from brown to black can be achieved on copper-rich alloys by treatment with potassium sulfide.
[3] Cobalt-containing alloys, e.g. 75% gold with 25% cobalt, form a black oxide layer with heat treatment at 700–950 °C.
A femtosecond laser pulse deforms the surface of the metal, creating an immensely increased surface area which absorbs virtually all the light that falls on it, thus rendering it deep black,[18] but this method is used in high technology applications rather than for appearance in jewelry.
The broadness of the plasmon resonance, and absorption wavelength range, depends on the interaction between different gold nanoparticles.