Combined injectable birth control
Combined injectable contraceptives (CICs) are a form of hormonal birth control for women.
They consist of monthly injections of combined formulations containing an estrogen and a progestin to prevent pregnancy.
CICs are different from progestogen-only injectable contraceptives (POICs), such as depot medroxyprogesterone acetate (DMPA; brand names Depo-Provera, Depo-SubQ Provera 104) and norethisterone enantate (NETE; brand name Noristerat), which are not combined with an estrogen and are given once every two to three months instead of once a month.
[2] Hormonal contraception works primarily by preventing ovulation, but it may also thicken the cervical mucus inhibiting sperm penetration.
[27] Conversely, combined oral contraceptive pills containing ethinylestradiol have considerable effects on coagulation and fibrinolysis.
[24] Progesterone derivatives including medroxyprogesterone acetate, algestone acetophenide (dihydroxyprogesterone acetophenide), hydroxyprogesterone caproate, and megestrol acetate are active themselves and are not prodrugs, whereas the testosterone derivative norethisterone enantate is a prodrug of norethisterone.
Because CICs are administered parenterally, they bypass the first-pass effect in the liver and intestines that occurs with oral administration of estrogens.
[28] CICs have antigonadotropic effects via their estrogenic and progestogenic activity and inhibit fertility and suppress sex hormone levels.
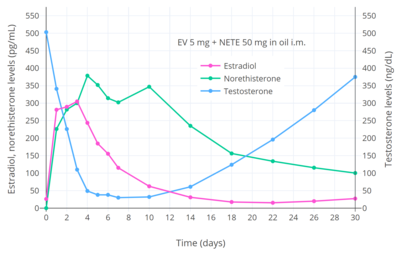
A single intramuscular injection of estradiol valerate/norethisterone enanthate (5 mg/50 mg) (Mesigyna) has been found to strongly suppress testosterone levels in men.
[60][14][15] On 5 October 2000, Pharmacia received FDA approval for Lunelle Monthly Contraceptive Injection.