Combustion
A simple example can be seen in the combustion of hydrogen and oxygen into water vapor, a reaction which is commonly used to fuel rocket engines.
A complete set of equations for the combustion of a hydrocarbon in the air, therefore, requires an additional calculation for the distribution of oxygen between the carbon and hydrogen in the fuel.
[4] Incomplete combustion will occur when there is not enough oxygen to allow the fuel to react completely to produce carbon dioxide and water.
In incomplete combustion, products of pyrolysis remain unburnt and contaminate the smoke with noxious particulate matter and gases.
They may be necessary to enable large combustion devices, such as thermal power stations, to reach legal emission standards.
An additional problem associated with nitrogen oxides is that they, along with hydrocarbon pollutants, contribute to the formation of ground level ozone, a major component of smog.
Exposure to moderate and high levels of carbon monoxide over long periods is positively correlated with the risk of heart disease.
People who survive severe carbon monoxide poisoning may suffer long-term health problems.
[8] Carbon monoxide from the air is absorbed in the lungs which then binds with hemoglobin in human's red blood cells.
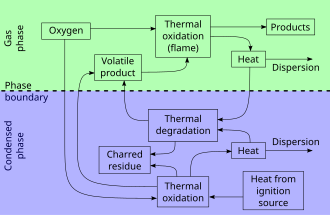
Smoldering is the slow, low-temperature, flameless form of combustion, sustained by the heat evolved when oxygen directly attacks the surface of a condensed-phase fuel.
Common examples of smoldering phenomena are the initiation of residential fires on upholstered furniture by weak heat sources (e.g., a cigarette, a short-circuited wire) and the persistent combustion of biomass behind the flaming fronts of wildfires.
Organic materials undergoing bacterial composting can generate enough heat to reach the point of combustion.
[9] Combustion resulting in a turbulent flame is the most used for industrial applications (e.g. gas turbines, gasoline engines, etc.)
In such an environment, the thermal and flow transport dynamics can behave quite differently than in normal gravity conditions (e.g., a candle's flame takes the shape of a sphere.[10]).
Microgravity combustion research contributes to the understanding of a wide variety of aspects that are relevant to both the environment of a spacecraft (e.g., fire dynamics relevant to crew safety on the International Space Station) and terrestrial (Earth-based) conditions (e.g., droplet combustion dynamics to assist developing new fuel blends for improved combustion, materials fabrication processes, thermal management of electronic systems, multiphase flow boiling dynamics, and many others).
Carbon becomes a stable phase at 1200 K and 1 atm pressure when z is less than 30% of the stoichiometric value, at which point the combustion products contain more than 98% H2 and CO and about 0.5% CH4.
The most common examples are natural gas, propane, kerosene, diesel, petrol, charcoal, coal, wood, etc.
The flash point of liquid fuel is the lowest temperature at which it can form an ignitable mix with air.
Unburned fuel (usually CO and H2) discharged from the system represents a heating value loss (as well as a safety hazard).
[23][24] Additional material and heat balances can be made to quantify the thermal advantage from preheating the combustion air,[25][26] or enriching it in oxygen.
The lowest-energy configuration of the dioxygen molecule is a stable, relatively unreactive diradical in a triplet spin state.
To initiate combustion, energy is required to force dioxygen into a spin-paired state, or singlet oxygen.
A lack of oxygen or other improperly designed conditions result in these noxious and carcinogenic pyrolysis products being emitted as thick, black smoke.
Detailed descriptions of combustion processes, from the chemical kinetics perspective, require the formulation of large and intricate webs of elementary reactions.
[29] For instance, combustion of hydrocarbon fuels typically involve hundreds of chemical species reacting according to thousands of reactions.
The inclusion of such mechanisms within computational flow solvers still represents a pretty challenging task mainly in two aspects.
As a result, the direct numerical simulation of turbulent reactive flows with heavy fuels soon becomes intractable even for modern supercomputers.
[30] Therefore, a plethora of methodologies have been devised for reducing the complexity of combustion mechanisms without resorting to high detail levels.
Examples are provided by: The kinetic modelling may be explored for insight into the reaction mechanisms of thermal decomposition in the combustion of different materials by using for instance Thermogravimetric analysis.
In rockets, such as the F1 used in the Saturn V program, instabilities led to massive damage to the combustion chamber and surrounding components.