Coordination complex
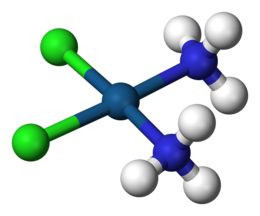
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents.
[1][2][3] Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes.
Originally, a complex implied a reversible association of molecules, atoms, or ions through such weak chemical bonds.
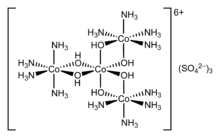
Werner discovered the spatial arrangements of the ligands that were involved in the formation of the complex hexacoordinate cobalt.
In 1911, Werner first resolved the coordination complex hexol into optical isomers, overthrowing the theory that only carbon compounds could possess chirality.
The number of bonds depends on the size, charge, and electron configuration of the metal ion and the ligands.
Large metals and small ligands lead to high coordination numbers, e.g. [Mo(CN)8]4−.
Due to their large size, lanthanides, actinides, and early transition metals tend to have high coordination numbers.
Most structures follow the points-on-a-sphere pattern (or, as if the central atom were in the middle of a polyhedron where the corners of that shape are the locations of the ligands), where orbital overlap (between ligand and metal orbitals) and ligand-ligand repulsions tend to lead to certain regular geometries.
The most observed geometries are listed below, but there are many cases that deviate from a regular geometry, e.g. due to the use of ligands of diverse types (which results in irregular bond lengths; the coordination atoms do not follow a points-on-a-sphere pattern), due to the size of ligands, or due to electronic effects (see, e.g., Jahn–Teller distortion): The idealized descriptions of 5-, 7-, 8-, and 9- coordination are often indistinct geometrically from alternative structures with slightly differing L-M-L (ligand-metal-ligand) angles, e.g. the difference between square pyramidal and trigonal bipyramidal structures.
[12] To distinguish between the alternative coordinations for five-coordinated complexes, the τ geometry index was invented by Addison et al.[16] This index depends on angles by the coordination center and changes between 0 for the square pyramidal to 1 for trigonal bipyramidal structures, allowing to classify the cases in between.
This system was later extended to four-coordinated complexes by Houser et al.[17] and also Okuniewski et al.[18] In systems with low d electron count, due to special electronic effects such as (second-order) Jahn–Teller stabilization,[19] certain geometries (in which the coordination atoms do not follow a points-on-a-sphere pattern) are stabilized relative to the other possibilities, e.g. for some compounds the trigonal prismatic geometry is stabilized relative to octahedral structures for six-coordination.
The arrangement of the ligands is fixed for a given complex, but in some cases it is mutable by a reaction that forms another stable isomer.
It is so called because the two isomers are each optically active, that is, they rotate the plane of polarized light in opposite directions.
In the first molecule shown, the symbol Λ (lambda) is used as a prefix to describe the left-handed propeller twist formed by three bidentate ligands.
The second molecule is the mirror image of the first, with the symbol Δ (delta) as a prefix for the right-handed propeller twist.
The electronic structure can be described by a relatively ionic model that ascribes formal charges to the metals and ligands.
Crystal field theory, introduced by Hans Bethe in 1929, gives a quantum mechanically based attempt at understanding complexes.
But crystal field theory treats all interactions in a complex as ionic and assumes that the ligands can be approximated by negative point charges.
Chemists tend to employ the simplest model required to predict the properties of interest; for this reason, CFT has been a favorite for the discussions when possible.
Thus, monomeric Ti(III) species have one "d-electron" and must be (para)magnetic, regardless of the geometry or the nature of the ligands.
In bi- and polymetallic complexes, in which the individual centres have an odd number of electrons or that are high-spin, the situation is more complicated.
In many cases these ligands are oxides or sulfides, but the metals are coordinated nonetheless, and the principles and guidelines discussed below apply.
The former are concerned primarily with polymeric structures, properties arising from a collective effects of many highly interconnected metals.
It is the equilibrium constant for its assembly from the constituent metal and ligands, and can be calculated accordingly, as in the following example for a simple case: where : x, y, and z are the stoichiometric coefficients of each species.
[32] This constant represents the reverse reaction for the decomposition of a complex ion into its individual metal and ligand components.
As a result of these complex ions forming in solutions they also can play a key role in solubility of other compounds.
So Kc, the new solubility constant, is denoted by: As metals only exist in solution as coordination complexes, it follows then that this class of compounds is useful in a wide variety of ways.
Synthetic coordination compounds are also used to bind to proteins and especially nucleic acids (e.g. anticancer drug cisplatin).
Qualitative inorganic analysis has largely been superseded by instrumental methods of analysis such as atomic absorption spectroscopy (AAS), inductively coupled plasma atomic emission spectroscopy (ICP-AES) and inductively coupled plasma mass spectrometry (ICP-MS).