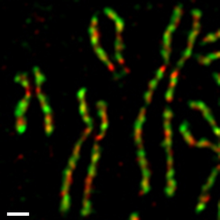
Condensin
Condensins are large protein complexes that play a central role in chromosome assembly and segregation during mitosis and meiosis (Figure 1).
[1][2] Their subunits were originally identified as major components of mitotic chromosomes assembled in Xenopus egg extracts.
Prokaryotic species also have condensin-like complexes that play an important role in chromosome (nucleoid) organization and segregation.
[18] Many eubacterial and archaeal species have SMC-ScpAB, whereas a subgroup of eubacteria (known as Gammaproteobacteria) including Escherichia coli has MukBEF.
[19] Despite highly divergent primary structures of their corresponding subunits between SMC-ScpAB and MukBEF, it is reasonable to consider that the two complexes play similar if not identical functions in prokaryotic chromosome organization and dynamics, based on their molecular architecture and their defective cellular phenotypes.
Recent studies report the occurrence of a third complex related to MukBEF (termed MksBEF) in some bacterial species.
[24] A recent cryo-EM study has shown that condensin undergoes large conformational changes that are coupled with ATP-binding and hydrolysis by its SMC subunits.
[33] On the other hand, fast-speed atomic force microscopy has demonstrated that the arms of an SMC dimer is far more flexible than was expected.
[39] It is postulated that this activity of condensin I helps fold DNA and promotes topoisomerase II-mediated resolution of sister chromatids.
[40] Early single-DNA-molecule experiments also demonstrated in real time that condensin I is able to compact DNA in an ATP-hydrolysis dependent manner.
Recent development of a reconstitution system has identified the histone chaperone FACT as an essential component of condensin I-mediated chromosome assembly in vitro, providing an important clue to this problem.
A fine-tuned balance between the actions of these non-SMC subunits could determine the differences in the rate of loop extrusion [47] and the activity of mitotic chromosome assembly [48][49][50][51] of the two complexes.
[51] Several attempts on mathematical modeling and computer simulation of mitotic chromosome assembly, based on molecular activities of condensins, have been reported.
On the other hand, condensin I is present in the cytoplasm during interphase, and gains access to chromosomes only after the nuclear envelope breaks down (NEBD) at the end of prophase.
Moreover, quantitative imaging analyses allow researchers to count the number of condensin complexes present on human metaphase chromosomes.
[75] In mice, requirements for condensin subunits in meiosis have been addressed by antibody-mediated blocking experiments[58] and conditional gene knockout analyses.
Recent studies have shown that condensins participate in a wide variety of chromosome functions outside of mitosis or meiosis.
It has been shown that the C-terminal tail of the CAP-D3 subunit is a major target for Cdk1 phosphorylation in the human condensin II complex.
[102] It was demonstrated that MCPH1, one of the proteins responsible for human primary microcephaly, has the ability to negatively regulate condensin II.
[14][59] A recent, comprehensive Hi-C study argues from an evolutionary point of view that condensin II acts as a determinant that converts post-mitotic Rabl configurations into interphase chromosome territories.