Controlled-release fertiliser
The slow-release properties depend on the degradation of the secondary sealant by soil microbes as well as mechanical imperfections (cracks, etc.)
Illustrating the problem, it is estimated that, on average, 16% of conventional nitrogen-based fertilizers is lost by evaporation (as NH3, N2O, N2) or run-off ammonia.
[4] Urease inhibitors, at levels of 0.05 weight percent, are added to urea-based fertilizers to control its conversion to ammonia.
[7] The rate of the release is determined by various main factors: (i) the low solubility of the compounds in the soil moisture, (ii) the breakdown of protective coating applied to fertilizer pellets, and (iii) the conversion of the chemicals into ammonia or similarly effective plant nutrient.
The fertiliser granules may have an insoluble substrate or a semi-permeable jacket that prevents dissolution while allowing nutrients to flow outward.
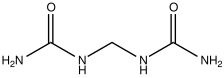
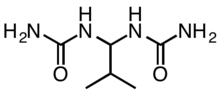
Isobutylidenediurea ("IBDU") and urea-formaldehyde slowly convert in the soil to urea, which is rapidly uptaken by plants.
"Multicote" is a process applying layers of low-cost fatty acid salts with a paraffin topcoat.
Recently, biodegradable polymers as coatings for slow/controlled-release fertilizer have attracted interest for their potential to increase fertilizer/pesticide utilization efficiency and reduce negative environmental effects.