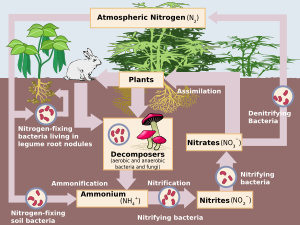
Nitrification
They are known for their ability to utilize ammonia as an energy source and are prevalent in a wide range of environments, such as soils, aquatic systems, and wastewater treatment plants.
AOB play a vital role in soil nitrification, making them key players in nutrient cycling.
They contribute to the transformation of ammonia derived from organic matter decomposition or fertilizers into nitrite, which subsequently serves as a substrate for nitrite-oxidizing bacteria (NOB).
However the discovery of Nitrososphaerota that are not obligate ammonia-oxidizers[13] complicates this conclusion,[14] as does one study that suggests that crenarchaeol may be produced by Marine Group II Euryarchaeota.
Nitrite oxidation is conducted by nitrite-oxidizing bacteria (NOB) from the taxa Nitrospirota,[16] Nitrospinota,[17] Pseudomonadota[18] and Chloroflexota.
Ammonia oxidation to nitrate in a single step within one organism was predicted in 2006[20] and discovered in 2015 in the species Nitrospira inopinata.
[23] In 1877, Jean-Jacques Schloesing and Achille Müntz, two French agricultural chemists working in Paris, proved that nitrification is indeed microbially mediated process by the experiments with liquid sewage and artificial soil matrix (sterilized sand with powdered chalk).
[24] Their findings were confirmed soon (in 1878) by Robert Warington who was investigating nitrification ability of garden soil at the Rothamsted experimental station in Harpenden in England.
[27] Although at that time, it was believed that two-step nitrification is separated into distinct life phases or character traits of a single microorganism.
Nitrosomonas europaea, as well as populations of soil-dwelling AOB, have been shown to assimilate the carbon dioxide released by the reaction to make biomass via the Calvin Cycle, and harvest energy by oxidizing ammonia (the other product of urease) to nitrite.
[32] In most environments, organisms are present that will complete both steps of the process, yielding nitrate as the final product.
The cost of this process resides mainly in aeration (bringing oxygen in the reactor) and the addition of an external carbon source (e.g., methanol) for the denitrification.
In distribution systems where chloramines are used as the secondary disinfectant, the presence of free ammonia can act as a substrate for ammonia-oxidizing microorganisms.
[34][35] Together with ammonification, nitrification forms a mineralization process that refers to the complete decomposition of organic material, with the release of available nitrogen compounds.
[36][37] The nitrification step of the cycle is of particular interest in the ocean because it creates nitrate, the primary form of nitrogen responsible for "new" production.
Several groups of ammonia-oxidizing bacteria (AOB) are known in the marine environment, including Nitrosomonas, Nitrospira, and Nitrosococcus.
[2][37] Subsequent metagenomic studies and cultivation approaches have revealed that some Thermoproteota (formerly Crenarchaeota) possess AMO.
Nitrification inhibitors are used widely, being added to approximately 50% of the fall-applied anhydrous ammonia in states in the U.S., like Illinois.
The inhibition of the nitrification process is primarily facilitated by the selection and inhibition/destruction of the bacteria that oxidize ammonia compounds.
The conversion of ammonia to hydroxylamine is the first step in nitrification, where AH2 represents a range of potential electron donors.
This is usually supplied by the compound hydroxylamine oxidoreductase (HAO) which catalyzes the reaction: The mechanism of inhibition is complicated by this requirement.
This method occurs by the inactivation of the enzyme via covalent modification of the product, which ultimately inhibits nitrification.
In particular, thiophosphoryl triamide has been a notable addition where it has the dual purpose of inhibiting both the production of urease and nitrification.
Nitrification is also thought to contribute to the formation of photochemical smog, ground-level ozone, acid rain, changes in species diversity, and other undesirable processes.
In addition, nitrification inhibitors have also been shown to suppress the oxidation of methane (CH4), a potent greenhouse gas, to CO2.
Both nitrapyrin and acetylene are shown to be potent suppressors of both processes, although the modes of action distinguishing them are unclear.