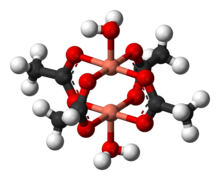
Copper(II) acetate
Anhydrous copper(II) acetate is a dark green crystalline solid, whereas Cu2(OAc)4(H2O)2 is more bluish-green.
[8][9][10][11] The two copper centers interact resulting in a diminishing of the magnetic moment such that at temperatures below 90 K, Cu2(OAc)4(H2O)2 is essentially diamagnetic.
Cu2(OAc)4(H2O)2 was a critical step in the development of modern theories for antiferromagnetic exchange coupling, which ascribe its low-temperature diamagnetic behavior to cancellation of the two opposing spins on the adjacent copper atoms.
A related reaction involving copper acetylides is the synthesis of ynamines, terminal alkynes with amine groups using Cu2(OAc)4.
It reacts with arsenic trioxide to form copper acetoarsenite, a powerful insecticide and fungicide called Paris green.