Copper(II) hydroxide
Cupric hydroxide is a strong base, although its low solubility in water makes this hard to observe directly.
It was produced on an industrial scale during the 17th and 18th centuries for use in pigments such as blue verditer and Bremen green.
The nature of the resulting copper(II) hydroxide however is sensitive to detailed conditions.
Often, when it is utilized for this purpose, it is prepared in situ by mixing a soluble copper(II) salt and potassium hydroxide.
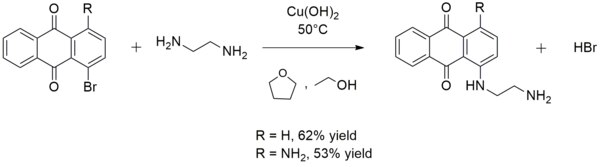
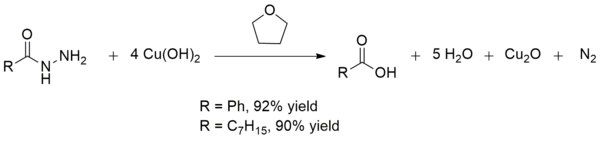
For example, copper(II) hydroxide catalyzes the reaction of ethylenediamine with 1-bromoanthraquinone or 1-amino-4-bromoanthraquinone to form 1-((2-aminoethyl)amino)anthraquinone or 1-amino-4-((2-aminoethyl)amino)anthraquinone, respectively:[15] Copper(II) hydroxide also converts acid hydrazides to carboxylic acids at room temperature.
Although other water-soluble copper compounds can be effective in this role, they generally result in high fish mortality.
Copper(II) hydroxide has been used as an alternative to the Bordeaux mixture, a fungicide and nematicide.
Copper(II) hydroxide has been combined with latex paint, making a product designed to control root growth in potted plants.
The rights are now owned by SePRO Corp.[17] It is now sold as Microkote either in a solution applied by the end user, or as treated pots.