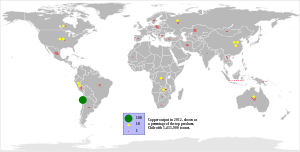
Copper extraction
Methods have evolved and vary with country depending on the ore source, local environmental regulations, and other factors.
[2] The earliest evidence of the cold-hammering of native copper comes from the excavation at Çayönü Tepesi in eastern Anatolia, which dates between 7200 to 6600 BCE.
Another find, at Shanidar Cave in Mergasur, Iraq, contained copper beads, and dates back to 8,700 BCE.
[5][6] The Pločnik archaeological site in southeastern Europe (Serbia) contains the oldest securely dated evidence of copper making at high temperature, from 5,000 BCE.
[7] The find in June 2010 extends for an additional 500 years, dated to 5th millennium BCE, representing the earlier record of copper smelting from Rudna Glava (Serbia).
[1] In froth flotation, the crushed ore is wetted, suspended in a slurry, and mixed with reagents that render the sulfide particles hydrophobic.
The air bubbles attach to the hydrophobic copper sulfide particles, which are conveyed to the surface where the froth is skimmed off.
The rock that has not floated off in the flotation cell is either discarded as tailings or further processed to extract other metals such as lead (from galena) and zinc (from sphalerite), should they exist.
Lime is used to raise the pH of the water bath, causing the collector to bond more efficiently to the copper sulfides.
Oxidised copper ores include carbonates such as azurite and malachite, the silicate chrysocolla, and sulfates such as atacamite.
[22] Processes including in situ, dump, and heap leaching are cost-effective methods that are suitable for extracting copper from low-grade ores.
Heap bioleaching presents a cost efficient extraction method that requires a less intensive energy input resulting in a higher profit.
[24] This extraction process can be applied to large quantities of low-grade ores, at a lower capital cost with minimal environmental impact.
[28][29] For oxide ores, solvent extraction and electrowinning technologies are used to recover the copper from the pregnant leach solution.
[30] Supergene sulfide ores rich in native copper are refractory to treatment with sulfuric acid leaching on all practicable time scales, and the dense metal particles do not react with froth flotation media.
This is because clay minerals interact with flotation reagents used in extraction processes, that are then consumed, which results in minimal recovery of a high grade copper concentrate.
[34] The purpose of the matte smelting stage is to eliminate as much of the unwanted iron, sulfur and gangue minerals (such as silica, magnesia, alumina and limestone) as possible, while minimizing the loss of copper.
[34] Copper sulfide and iron oxide can mix, but when sufficient silica is added, a separate slag layer is formed.
[44] In the case of calcine-charged furnaces, a significant portion of the sulfur has been eliminated during the roasting stage, and the calcine consists of a mixture of copper and iron oxides and sulfides.
[44] The main equilibration reaction is: The slag and the matte form distinct layers that can be removed from the furnace as separate streams.
[44] The matte taphole is normally a hole through a water-cooled copper block that prevents erosion of the refractory bricks lining the furnace.
Refining is achieved by electrolysis, which exploits the easy (low potential) and selective conversion of copper(II) solutions to the metal.
More noble metals and less soluble elements such as silver, gold, selenium, and tellurium settle to the bottom of the cell as anode slime, which forms a salable by-product.
At the cathode (reduction reaction), Cu2+ ions are reduced in copper metal and Cu(s) plates out, but less noble constituents such as arsenic and zinc remain in solution unless a higher voltage is used.
The customer in this case can be a smelter, who on-sells blister copper ingots to a refiner, or a smelter-refiner which is vertically integrated.
[54] The typical contract for a miner is denominated against the London Metal Exchange price, minus the TC-RCs and any applicable penalties or credits.
Penalties may be assessed against copper concentrates according to the level of deleterious elements such as arsenic, bismuth, lead or tungsten.
The chemical specification for electrolytic grade copper is ASTM B 115-00 (a standard that specifies the purity and maximum electrical resistivity of the product).
[82] Based on discovery rates and existing geologic surveys, researchers estimated in 2006 that 1.6 billion metric tons of copper could be brought into use.
Yet, despite the material being far less widespread, the cost of, for example, a copper pot was vastly lower in the late 20th century than 5000 years ago.