Electrometallurgy
Electrometallurgy is a method in metallurgy that uses electrical energy to produce metals by electrolysis.
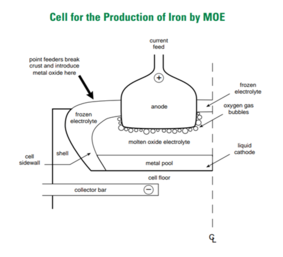
There are seven categories of these processes: Molten Oxide Electrolysis in steelmaking is utilizing electrons as the reducing agent instead of coke as in conventional blast furnace.
For steel production, this method uses an inert anode (Carbon, Platinum, Iridium or Chromium-based alloy)[4] and places iron ore in the cathode.
[6] The electrolysis reaction will produce molten pure iron as a main product and oxygen as its by-product.
Currently Massachusetts-based Boston Metal company is in a process to scale up this technology to an industrial level.
This process is suitable for secondary steelmaking industry which recycling steel scrap that has variety of carbon content in their feedstock.
[7] This method aim to replace current conventional method that utilizing Basic Oxygen Furnace (BOF) to reduce carbon content of iron by blowing oxygen to make it react with carbon and forming CO2.
In electrorefining, decarburization process happened in electrochemical cell that composed of inert electrode, slag and steel.
Oxygen ion from slag decompose and oxidize carbon on steel and to form CO. That decarburizing reaction is occurred in three steps as follow.
[7] (ads) means adsorbed intermediate The total reaction from this cell is following this scheme[7]