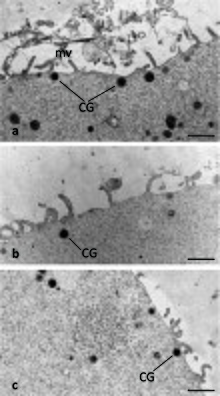
Cortical granule
In mammals, the oocyte's extracellular matrix includes a surrounding layer of perivitelline space, zona pellucida, and finally cumulus cells.
Experimental evidence has demonstrated that the released contents of the cortical granules modify the oocyte's extracellular matrix, particularly the zona pellucida.
[1] In addition to modifying the oocyte's extracellular matrix and establishing a block to polyspermy, the exocytosis of cortical granules may also contribute towards protection and support of the developing embryo during preimplantation.
More specifically, in the human, monkey, hamster, and rabbit, cortical granules are established once the ovarian follicle is multilayered.
During the early stages of oocyte growth, the Golgi complex increases in size, proliferates, and produces small vesicles that migrate to the cell's subcortical region.
These small vesicles will fuse with one another to form mature cortical granules, which are thus established as separate entities from the Golgi.
It has been shown in both mammalian and non-mammalian animal models that cortical granule migration depends on cytoskeleton processes, particularly microfilament activity.
Studies with rodent oocytes suggest that certain cortical granules undergo redistribution and/or exocytosis throughout the meiotic cycle thus establishing the CGFDs.
Additionally, some pre-fertilization cortical granule exocytotic events occur in the cell's cleavage furrow simultaneously with polar body formation.
For example, in the fertilized sea urchin egg, it has been shown that the cortical granule exocytosis immediately follows the calcium increase after approximately 6 seconds.
Furthermore, when calcium waves were suppressed experimentally, cortical granule exocytosis and/or alterations in the extracellular matrix did not occur.
As demonstrated in unfertilized vertebrate oocytes, cortical granule exocytosis is induced when calcium is artificially increased.
Researchers have suggested that calreticulin serves as a chaperone protein for other cortical granule components contributing to polyspermy prevention.
Additionally contributing to polyspermy prevention, calreticulin may also inhibit certain glycoproteins, which promote interaction between the oocyte and sperm.
Furthermore, upon exocytosis, this calreticulin interacts with the oocyte's cytoskeleton, thereby allowing the transmission of transmembrane signaling for the continuance of the cell's cycle.
Upon release from the cortical granule, p32 appears to either function briefly or undergo a modification shortly after fertilization because only small amounts of p32 are present on the embryo.