Endoplasmic reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryotic cell, and has many other important functions such as protein folding.
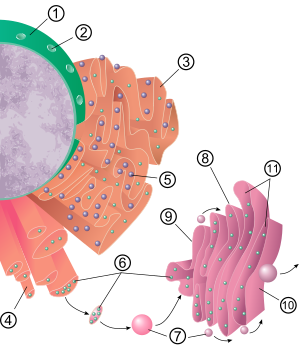
The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER.
The SER lacks ribosomes and functions in lipid synthesis but not metabolism, the production of steroid hormones, and detoxification.
[2][3] The lacy membranes of the endoplasmic reticulum were first seen by electron microscopy in 1945 by Keith R. Porter, Albert Claude, and Ernest F.
This special complex forms when a free ribosome begins translating the mRNA of a protein destined for the secretory pathway.
[10] The membrane of the rough endoplasmic reticulum is in the form of large double-membrane sheets that are located near, and continuous with, the outer layer of the nuclear envelope.
[12][13] Although there is no continuous membrane between the endoplasmic reticulum and the Golgi apparatus, membrane-bound transport vesicles shuttle proteins between these two compartments.
COPII targets vesicles to the Golgi apparatus and COPI marks them to be brought back to the rough endoplasmic reticulum.
The rough endoplasmic reticulum works in concert with the Golgi complex to target new proteins to their proper destinations.
Cells which secrete these products, such as those in the testes, ovaries, and sebaceous glands have an abundance of smooth endoplasmic reticulum.
Smooth endoplasmic reticulum is found in a variety of cell types (both animal and plant), and it serves different functions in each.
[citation needed] The sarcoplasmic reticulum (SR), from the Greek σάρξ sarx ("flesh"), is smooth ER found in muscle cells.
The only structural difference between this organelle and the smooth endoplasmic reticulum is the composition of proteins they have, both bound to their membranes and drifting within the confines of their lumens.
[23][24] After their release from the sarcoplasmic reticulum, calcium ions interact with contractile proteins that utilize ATP to shorten the muscle fiber.
Proteins that are transported by the endoplasmic reticulum throughout the cell are marked with an address tag called a signal sequence.
The imported ATP is vital for the ER to carry out its house keeping cellular functions, such as for protein folding and trafficking.
[38] Abnormalities in XBP1 lead to a heightened endoplasmic reticulum stress response and subsequently causes a higher susceptibility for inflammatory processes that may even contribute to Alzheimer's disease.
[41] The UPR is activated in response to an accumulation of unfolded or misfolded proteins in the lumen of the endoplasmic reticulum.