Trimethoprim/sulfamethoxazole
[2] Trimethoprim/sulfamethoxazole (TMP/SMX) is the medicine most commonly used to prevent Pneumocystis jirovecii pneumonia (PCP)[12] People who get Pneumocystis pneumonia have a medical condition that weakens their immune system, like HIV/AIDS, or take medicines (such as corticosteroid, monoclonal antibody and immunosuppressants) that reduce the body's ability to fight bacterial and viral infections.
[15][16] Its use during pregnancy is contraindicated, although it has been placed in Australian pregnancy category C.[13] Its use during the first trimester (during organogenesis) and 12 weeks prior to pregnancy has been associated with an increased risk of congenital malformations, especially malformations associated with maternal folic acid deficiency (which is most likely related to the mechanism of action of co-trimoxazole) such as neural tube defects such as spina bifida, cardiovascular malformations (e.g. Ebstein's anomaly), urinary tract defects, oral clefts, and club foot in epidemiological studies.
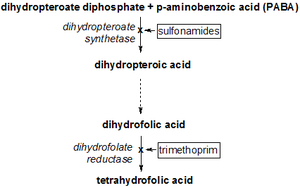
[25][26][27] Trimethoprim and sulfamethoxazole have a greater effect when given together than when given separately, because they inhibit successive steps in the folate synthesis pathway.
[14] Sulfamethoxazole, a sulfonamide, induces its therapeutic effects by interfering with the de novo (that is, from within the cell) synthesis of folate inside microbial organisms such as protozoa, fungi and bacteria.
[14] Trimethoprim serves as a competitive inhibitor of dihydrofolate reductase (DHFR), hence inhibiting the de novo synthesis of tetrahydrofolate, the biologically active form of folate.
[14] Tetrahydrofolate is crucial in the synthesis of purines, thymidine, and methionine which are needed for the production of DNA and proteins[28] during bacterial replication.
[citation needed] The generic British Approved Name (BAN) Co-trimoxazole is used for trimethoprim/sulfamethoxazole manufactured and sold by many different companies.