Click chemistry
[1][2] In this seminal paper, Sharpless argued that synthetic chemistry could emulate the way nature constructs complex molecules, using efficient reactions to join together simple, non-toxic building blocks.
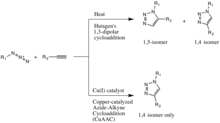
The copper(I)-catalysis of the Huisgen 1,3-dipolar cycloaddition was discovered concurrently and independently by the groups of Valery V. Fokin and K. Barry Sharpless at the Scripps Research Institute in California[16] and Morten Meldal in the Carlsberg Laboratory, Denmark.
[27][28][29] Although the Cu(I)-catalyzed variant was first reported by Meldal and co-workers for the synthesis of peptidotriazoles on solid support, their conditions were far from the true spirit of click chemistry and were overtaken by the publicly more recognized Sharpless.
Meldal and co-workers also chose not to label this reaction type "click chemistry" which allegedly caused their discovery to be largely overlooked by the mainstream chemical society.
An analogous RuAAC reaction catalyzed by ruthenium, instead of copper, was reported by the Jia and Fokin groups in 2005, and allows for the selective production of 1,5-isomers.
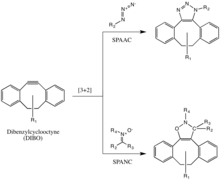
But cyclooctyne derivatives such as DIFO, dibenzylcyclooctyne (DIBO or DBCO) and biarylazacyclooctynone (BARAC) have all been used successfully in the SPAAC reaction to probe for azides in living systems.
[33][34][35] Diaryl-strained-cyclooctynes including dibenzylcyclooctyne (DIBO) have also been used to react with 1,3-nitrones in strain-promoted alkyne-nitrone cycloadditions (SPANC) to yield N-alkylated isoxazolines.
Trans-cycloalkenes (usually cyclooctenes) and other strained alkenes such as oxanorbornadiene react in click reactions with a number of partners including azides, tetrazines and tetrazoles.
The activated double bond in oxanobornadiene makes a triazoline intermediate that subsequently spontaneously undergoes a retro Diels-alder reaction to release furan and give 1,2,3- or 1,4,5-triazoles.
[40] Strained cyclooctenes and other activated alkenes react with tetrazines in an inverse electron-demand Diels-Alder followed by a retro [4+2] cycloaddition (see figure).
[43] Finally, the non-fluorogenic reactants give rise to a fluorogenic product, equipping the reaction with a built-in spectrometry handle.
[44] The criteria for click reactions are designed to make the chemistry biocompatible, for applications like isolating and targeting molecules in complex biological environments.
In many applications, click reactions join a biomolecule and a reporter molecule or other molecular probe, a process called bioconjugation.
[45] The possibility of attaching fluorophores and other reporter molecules has made click chemistry a very powerful tool for identifying, locating, and characterizing both old and new biomolecules.. One of the earliest and most important methods in bioconjugation was to express a reporter gene, such as the gene green fluorescent protein (GFP), on the same genetic sequence as a protein of interest.
Additionally, using this method, GFP can only be attached to proteins, leaving other important biomolecular classes (nucleic acids, lipids, carbohydrates, etc.)
To overcome these challenges, chemists have opted to proceed by identifying pairs of bioorthogonal reaction partners, thus allowing the use of small exogenous molecules as biomolecular probes.
By developing specific and controllable bioorthogonal reactions, scientists have opened up the possibility of hitting particular targets in complex cell lysates.
These techniques represent a part of the field of chemical biology, in which click chemistry plays a fundamental role by intentionally and specifically coupling modular units to various ends.
[49] This approach has been used in numerous studies, and discoveries include that salinomycin localizes to lysosomes to initiate ferroptosis in cancer stem cells[50] and that metformin derivatives accumulate in mitochondria to chelate copper(II), affecting metabolism and epigenetic changes downstream in inflammatory macrophages.
For example, an UAA with an azide side group provides convenient access for cycloalkynes to proteins tagged with this "AHA" unnatural amino acid.
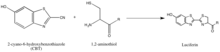
[55] The synthesis of luciferin exemplifies another strategy of isolating reaction partners, which is to take advantage of rarely-occurring, natural groups such as the 1,2-aminothiol, which appears only when a cysteine is the final N' amino acid in a protein.
[63] Licensees include Invitrogen,[64] Allozyne,[65] Aileron,[66] Integrated Diagnostics,[67] and the biotech company baseclick,[68] a BASF spin-off created to sell products made using click chemistry.
[69] Moreover, baseclick holds a worldwide exclusive license for the research and diagnostic market for the nucleic acid field.