Cubane
[4] Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles".
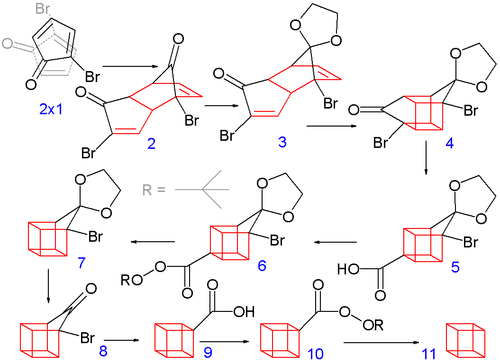
Treating this compound with diethylamine in diethyl ether causes elimination of two equivalents of hydrogen bromide to give the diene product.
The bromoketone group is converted to ring-contracted carboxylic acid 5 in a Favorskii rearrangement with potassium hydroxide.
A more approachable laboratory synthesis of disubstituted cubane involves bromination of the ethylene ketal of cyclopentanone to give a tribromocyclopentanone derivative.
[11] The synthesis of the octaphenyl derivative from tetraphenylcyclobutadiene nickel bromide by Freedman in 1962 pre-dates that of the parent compound.
[21] Cubene (1,2-dehydrocubane) and 1,4-cubanediyl(1,4-dehydrocubane) are enormously strained compounds which both undergo nucleophilic addition very rapidly, and this has enabled chemists to synthesize cubylcubane.
[22] Chemists at the University of Chicago extended and modified the sequence in a way that permits the preparation of a host of [n]cubylcubane oligomers.
[23] The [n]cubylcubanes are rigid molecular rods with the particular promise at the time of making liquid crystals with exceptional UV transparency.
In contrast, researchers at Penn State University showed that poly-cubane synthesized by solid-state reaction is 100% sp3 carbon bonded with a tetrahedral angle (109.5°) and exhibits exceptional optical properties (high refractive index).