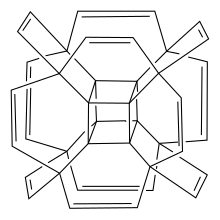
Hypercubane
Hypercubane was first proposed in 2014 by Pichierri and studied computationally by density functional theory.
To facilitate the future hypercubane spectroscopic identification chemical shifts for both 13C and 1H NMR-active nuclei have been calculated by Pichierri.
[1] Two years later, in 2016, studying the pyrolysis of hypercubane by means of tight-binding molecular dynamics simulations, Maslov and Katin demonstrated that hypercubane possessed high thermal stability comparable with the classic cubane C8H8.
[2] It was shown that hypercubane lifetime at room temperature tended to infinity.
Among the possible hypercubane decomposition products at high temperatures (more than 1000 K) one can observe polycyclic airscrew-like hydrocarbon C34H18 based on three combined graphene fragments passivated by hydrogen atoms and three isolated acetylene molecules.