Curium(III) chloride
Curium(III) chloride is the chemical compound with the formula CmCl3.
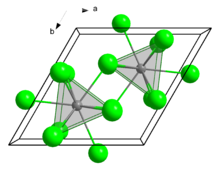
Curium(III) chloride has a 9 coordinate tricapped trigonal prismatic geometry.
[1] Curium(III) chloride can be obtained from the reaction of hydrogen chloride gas with curium dioxide, curium(III) oxide, or curium(III) oxychloride at a temperature of 400-600 °C: It can also be obtained from the dissolution of metallic curium in dilute hydrochloric acid:[2] This method has a number of disadvantages associated with the ongoing processes of hydrolysis and hydration of the resulting compound in an aqueous solution, making it problematic to obtain a pure product using this reaction.
It can be obtained from the reaction of curium nitride with cadmium chloride:[3]
You can help Wikipedia by expanding it.This inorganic compound–related article is a stub.